Publisher
India Pharma Outlook
published at
September 17, 2025
Understanding QA and QC Failures in Pharma
In pharmaceutical manufacturing,QA (Quality Assurance) and QC (Quality Control) are the backbone of product integrity and regulatory compliance. When either fails, the consequences can be severe - from regulatory action to patient harm.
QA failures in pharma often stem from poor oversight, weak quality systems, or a lack of documentation control. QA is responsible for ensuring that all processes are properly designed, validated, and compliant with GMP (Good Manufacturing Practices). A failure in QA is typically systemic - such as inadequate SOPs, poor deviation management, or non-functional CAPA systems.
QC failures in pharma, on the other hand, usually arise from errors in product testing, lab documentation, or equipment calibration. QC is process-specific - focused on ensuring that finished products meet quality specifications before release. Failures here can include out-of-specification (OOS) results not properly investigated or incorrect test methods being used.
Together, QA and QC failures contribute heavily to GMP non-conformance examples, which are often flagged during inspections as part of FDA 483 observations. These issues may appear small - a missing signature, a skipped calibration - but they reflect deeper cracks in a company's quality culture.
Most pharma teams know QA and QC are essential, but the line between them often gets blurred. This confusion is one of the biggest hidden drivers of GMP non-compliance, FDA 483s, and costly batch rejections.
👉 To make it crystal clear, here’s a short explainer video that breaks down:
- Why QA = prevention
- Why QC = detection
- How misalignment creates compliance gaps
- And how Inotek helps close the QA–QC loop
Common Deviations in Pharma QA/QC
Across FDA inspections, certain types of deviations appear again and again - highlighting weak spots in pharma quality systems. These common deviations in pharma QA/QC are not only frequent but also preventable with the right systems and training.
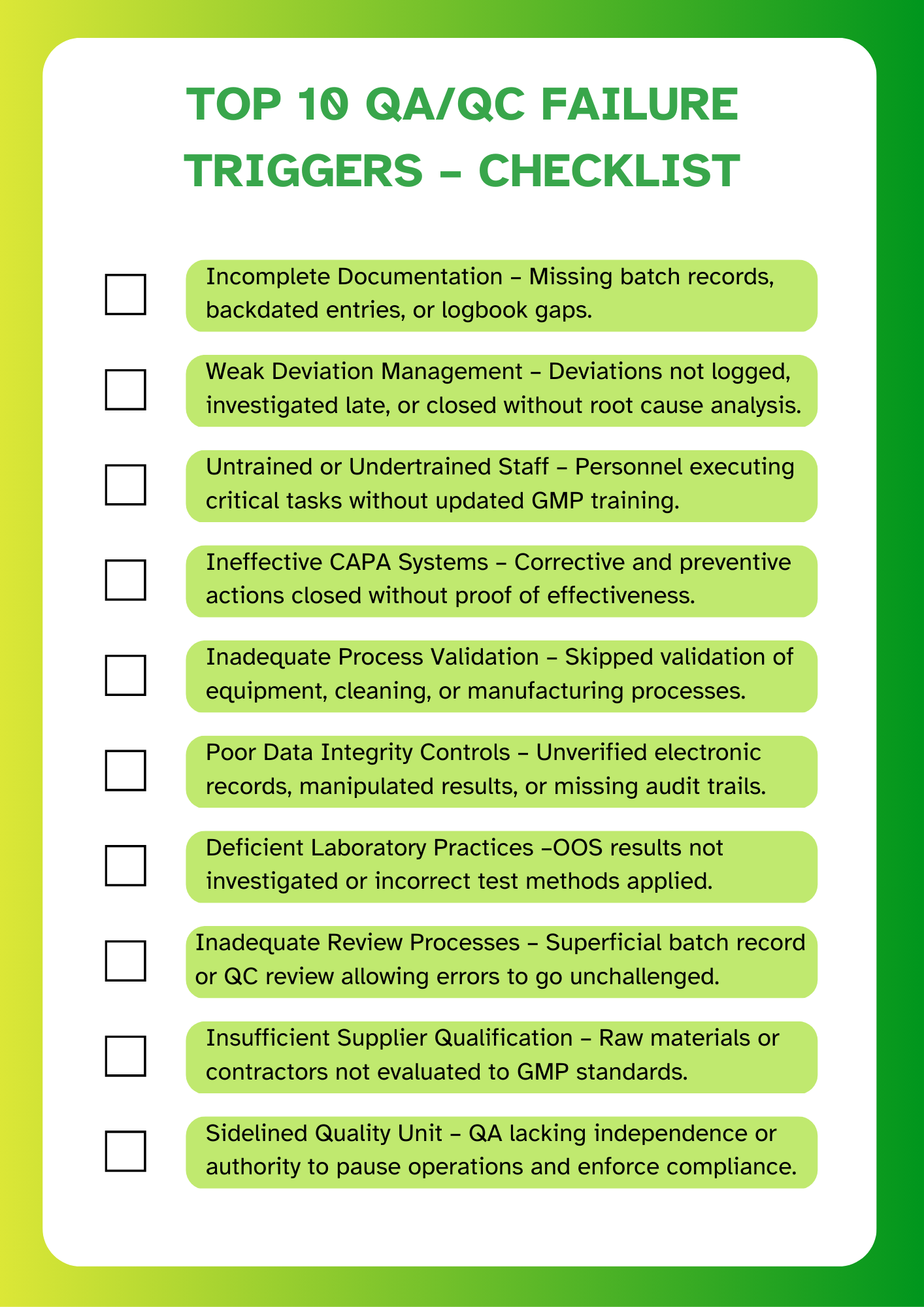
- Documentation Gaps: Missing batch records, incomplete logbooks, or backdated entries are among the most cited issues in FDA audit findings in the pharma industry. These raise red flags about data integrity and traceability.
- Untrained or Poorly Trained Personnel: Staff executing critical operations without updated training is a major compliance risk. It points to weak QA oversight and can lead to both QA and QC failures in pharma.
- Deviation Mishandling: Deviations that are not logged, investigated late, or closed without proper root cause analysis are one of the clearest indicators of non-compliant quality systems.
- Inadequate Review Processes: Quality checks often fail when batch records or lab reports are reviewed superficially - especially when errors or anomalies go unchallenged.
Each of these issues might seem minor on its own, but together, they show systemic quality failure and expose companies to FDA 483 observations and long-term pharma compliance risks.
Root Causes of QA/QC Deviations
Behind every deviation is a root cause - and understanding these is key to building a compliant and resilient pharma operation.
In many cases, the QA/QC deviation root cause isn’t a one-off mistake, but a reflection of deeper system weaknesses:
- Weak Investigations: Many firms lack robust deviation investigation procedures. This leads to recurring issues because the real cause is never addressed.
- Ineffective CAPA (Corrective and Preventive Action): CAPAs are often poorly designed, poorly tracked, or closed without evidence of effectiveness. This creates a false sense of resolution.
- Insufficient Process Validation: Skipping or rushing through validation (process, equipment, cleaning) can result in unpredictable outcomes that manifest as QC failures in pharma operations.
A Pareto analysis of FDA warning letters shows that 82% of critical observations fall into just four areas:
1. Quality Systems
2. Data Integrity
3. Laboratory Controls
4. Production & Process Controls
These are the systemic drivers of GMP non-conformance - and every pharma company or EPC partner must treat them as priority risk zones.
Before looking at the financial impact, it’s important to recognise the most common triggers of QA/QC failures in pharma. Below is a quick checklist based on recurring FDA 483 observations.
The Financial and Compliance Impact of QA/QC Failures
QA failures in pharma and QC failures in pharma don’t just lead to regulatory warnings - they carry significant financial and operational costs. Whether it's a missed deviation, poor documentation, or equipment failure, the ripple effect can disrupt production, delay approvals, and damage trust.
Beyond immediate penalties, these failures trigger long-term compliance issues that affect market access, product confidence, and future inspections. The impact of QA/QC failures on compliance also includes:
- Risk of repeat observations in FDA 483s
- Warning letters or import alerts
- Delayed product launches or withdrawn ANDAs
- Stricter oversight during follow-up inspections
The cost of QA failures in the pharma industry is often underestimated. What appears as a minor deviation can quickly escalate into a multi-million dollar remediation programme - especially if the root cause was ignored or left unaddressed.
In regulated environments, non-compliance costs far more than prevention. Addressing failures late means higher consulting fees, production halts, batch rework, and reputational damage - all of which are avoidable with robust QA/QC systems in place.
Direct Costs of QA/QC Failures
The immediate financial hit from QA or QC failures is often sharp and unavoidable. These direct costs can cripple operations, delay product delivery, and demand urgent resource allocation.
Here’s where pharma companies typically feel the burn:
- Remediation Expenses: After an FDA 483, firms may need to revalidate processes, rewrite SOPs, upgrade equipment, or invest in external audits - often running into hundreds of thousands of dollars.
- Consulting and Legal Fees: Engaging GMP consultants, legal teams, or ex-FDA experts for response drafting and remediation planning is costly - especially if the 483 involves data integrity or manufacturing controls.
- Production Downtime: Halting production to investigate deviations or retrain staff can result in lost batches, missed shipments, and contract penalties.
- Retraining Costs: A single QA or QC failure may require company-wide retraining on documentation practices, deviation reporting, or lab protocols.
These costs escalate quickly when companies don’t have proactive QA systems in place - especially during facility expansion, retrofits, or scale-up phases.
Indirect and Long-Term Costs
While direct costs from QA and QC failures in pharma are immediately visible, the indirect consequences can be far more damaging and harder to recover from.
Here are the most critical long-term cost drivers:
- Product Recalls: If quality deviations go undetected until after release, recalls can cost millions - not just in logistics and disposal, but in regulatory scrutiny and brand damage.
- Reputation Loss: Once a firm is associated with quality lapses or FDA audit findings, regaining market and partner trust becomes an uphill battle - especially for CDMOs and export-driven companies.
- Consent Decrees and Warning Letters: Escalated enforcement actions can lead to long-term manufacturing suspensions, third-party oversight, and loss of supply contracts.
- Opportunity Cost: When teams are tied up responding to failures, new product development, facility expansion, and business growth take a backseat.
These issues represent serious pharma compliance risks that often go unquantified. But the reality is simple: remediation costs more than readiness.
In fact, retrospective analysis shows that many firms spend 2–3X more on fixing issues than they would have spent building a compliance-first QMS from the beginning.
Benchmarking Cost Examples
Real-world benchmarks show just how costly QA/QC failures in pharma can be - and how much can be saved through quality-first practices.
- $1.2 Million Saved through “Right First Time” (RFT) process improvements that reduced deviation frequency and investigation time
- $5 Million Preserved by identifying and resolving a recurring OOS trend during internal audits before FDA inspection
- $12 Million Avoided in remediation by implementing robust CAPA effectiveness tracking tied to real-time quality metrics
These numbers aren’t hypothetical - they come from documented pharma case studies. They prove a critical point:
💡 Proactive QA investment saves far more than reactive cleanup.
Pharma firms and their EPC partners must treat GMP compliance as a core business metric - not just a regulatory requirement.
FDA 483 Observations and Escalation Risks
FDA 483 observations are not just inspection findings - they are early warnings of systemic risk. If not handled properly, these observations can lead to warning letters, import alerts, or even consent decrees.
The FDA audit findings that pharma companies face typically fall into key categories:
- Inadequate quality systems
- Poor documentation practices
- Incomplete investigations
- Laboratory data integrity issues
Each 483 observation reflects a deviation from GMP standards. But when observations are repeated across inspections - or across multiple sites - it signals deep-rooted QMS failures.
Understanding how to interpret, respond to, and prevent these observations is essential. For EPC partners like Inotek, this also means designing facilities and validation frameworks that anticipate and eliminate known 483 triggers before operations begin.
📌 483s don’t stay internal. Their content is often reviewed by business partners, global regulators, and investors.
Failure to respond comprehensively can escalate quickly - both in terms of regulatory enforcement and business impact.
From FDA 483 to Warning Letters
An FDA 483 observation is a red flag - but a warning letter is a formal regulatory action. The transition from one to the other usually happens when:
- The 483 observations are serious, repetitive, or show a lack of control over GMP processes.
- The company’s response to the 483 is incomplete, defensive, or lacks real corrective action.
- The FDA determines the site poses a risk to product quality or patient safety.
The escalation path typically looks like this:
1. FDA 483 - Identifies objectionable conditions during inspection.
2. Company Response - Expected within 15 business days; must show genuine intent to fix issues.
3. FDA Review - If the response is inadequate or issues are severe...
4. Warning Letter - Publicly posted; signals significant regulatory concern.
5. Consent Decree or Import Alert - In cases of continued non-compliance or serious risk.
📉 Lessons from FDA warning letters show that most companies miss the mark in three key areas:
- Not identifying true root causes
- Not implementing sustainable CAPAs.
- Not verifying CAPA effectiveness over time.
The message is clear: A poor 483 response is more dangerous than the 483 itself.
For pharma-construction partners like Inotek, this means integrating response-readiness into facility design, documentation systems, and operational SOPs - so that future 483s don’t escalate at all.
Case Study: Ranbaxy’s $500M Fallout
One of the most well-known examples of QA/QC failure in the pharma industry is the case of Ranbaxy Laboratories - a cautionary tale that still shapes FDA oversight today.
🧾 What happened:
- Ranbaxy was found to have falsified data, manipulated lab test results, and submitted unverified information to regulators.
- Investigations revealed systemic data integrity issues, a lack of audit trails, and poor QA oversight across multiple sites.
- Despite repeated warnings, the company failed to implement effective corrective actions.
📉 The outcome:
- Multiple sites were banned from exporting to the U.S.
- The brand suffered irreversible reputational damage, eventually leading to acquisition and operational downsizing.
This case illustrates:
- How FDA 483 observations, when ignored, can snowball into criminal investigations and massive financial penalties.
- The importance of having compliance-first systems, strong QA leadership, and real-time quality monitoring.
- Why global pharma firms now demand FDA-ready design and validation from EPC partners right from day one.
💡 Ranbaxy didn’t fail because of a single mistake. It failed because its quality culture tolerated small failures - until they exploded.
Strategic Risk Mitigation in Pharma QA
Preventing QA failures in pharma requires more than reactive fixes. It demands a strategic, system-wide approach to risk identification, response, and prevention.
Effective risk mitigation focuses on:
- Strengthening quality governance structures
- Investing in real-time monitoring and deviation tracking
- Standardising global QMS frameworks
- Anticipating regulatory triggers seen in previous FDA 483 observations
💡 The goal isn’t just to pass inspections - it’s to build resilience.
For pharma construction project management companies like Inotek, this means embedding quality safeguards from the earliest phases of project planning - including:
- Compliance-first layouts to minimise cross-contamination and human error
- Validation-ready documentation and equipment design
- Infrastructure that supports data integrity by design
Ultimately, proactive QA planning reduces remediation costs, protects product timelines, and builds trust with both regulators and partners.
Inotek: Your Strategic Partner in Compliance-First Pharma Project Execution
The complexities of managing QA/QC risks and avoiding costly FDA 483 observations demand specialised expertise and compliance-focused execution. This is where Inotek steps in as your strategic partner.
We don’t just build pharma facilities; we engineer compliance, resilience, and cost-efficiency into every project—aligned with global regulatory standards like FDA, EMA, WHO, and CDSCO.
Our Comprehensive Approach Includes:
- GMP-First Facility Design: Layouts that minimise deviations, cross-contamination risks, and human error.
- Validation-Ready Infrastructure: Pre-engineered systems that reduce remediation costs and accelerate approval timelines.
- Digital QMS Integration: Documentation and audit trail systems designed to withstand FDA scrutiny.
- Lifecycle QA Support: From conceptual design to commissioning and CQV, ensuring inspection-readiness at every stage.
Proven Client Outcomes
By partnering with Inotek, pharma manufacturers have achieved:
- Faster facility commissioning and reduced downtime.
- Significant reduction in repeat deviations and CAPAs.
- Stronger inspection outcomes and enhanced market access.
Beyond Compliance: Future-Ready Facilities
While regulatory compliance forms the foundation, successful pharma project execution must also address evolving industry expectations around:
- Data integrity and digital compliance
- Scalable designs for global supply chain resilience
At Inotek, we ensure your facility is not just audit-ready, but engineered for long-term operational excellence and cost savings.
Recognised among the Top 10 Pharma Turnkey Contractors & Project Consultants in 2022 & 2025, Inotek helps pharma leaders design, build, and upgrade facilities that meet the strictest GMP and sustainability standards.
📞 Connect with our experts today or visitwww.inotek.co.in to schedule a consultation with Mr. Rohit Ochaney.
Whether you're planning a greenfield facility or optimising an existing setup, Inotek ensures your project is compliant, resilient, and future-proof.
FAQs
What are common QA and QC failures in pharma?
Common failures include incomplete documentation, poor deviation handling, inadequate CAPAs, and lack of validation, often cited in FDA 483 observations.
How do FDA 483 observations affect pharma companies?
FDA 483s highlight GMP violations and can escalate to warning letters or consent decrees, resulting in halted production and reputational damage.
What is the cost of QA failures in the pharma industry?
Direct costs range from $250K to $5M per incident. Indirect costs include recalls, lost revenue, regulatory scrutiny, and long-term compliance risks.
How can pharma companies prevent QA/QC deviations?
By strengthening QA oversight, tracking quality metrics, ensuring CAPA effectiveness, and proactively using audit insights to improve QMS maturity.
How does Inotek help reduce pharma compliance risks?
Inotek builds compliance-first facilities with validation-ready systems, risk-mitigated layouts, and audit-supportive infrastructure to prevent future 483s.