Publisher
India Pharma Outlook
published at
April 10, 2025
Quality Assurance vs. Quality Control in GMP: Key Differences
Is your pharmaceutical company struggling with product quality inconsistencies or facing regulatory hurdles? Ensuring compliance with Good Manufacturing Practices (GMP) is critical to maintaining product safety, efficacy, and reputation. Many companies struggle to tell the difference between QA and QC. Quality Assurance is all about preventing mistakes by following organized processes, while Quality Control checks the final product to make sure it meets high-quality standards.
This article unpacks the QA and QC differences, their roles in pharmaceutical manufacturing, common challenges, and best practices to optimize quality control to quality assurance transition and regulatory compliance.
What is Quality Assurance (QA)?
Quality Assurance (QA) is all about making sure pharmaceutical products are made the right way from the start, instead of fixing mistakes later. It’s a process-focused approach that helps prevent defects by setting up structured workflows, keeping track of everything in detail, and managing risks to meet GMP quality. Basically, QA ensures that quality is built into every step of production so there’s less chance of things going wrong.
Core Responsibilities of QA
Quality Assurance (QA) plays a crucial role in ensuring products meet high standards before they reach consumers. It focuses on preventing mistakes rather than just fixing them after they happen.
- Risk Management: Identifying possible quality issues and finding ways to reduce or eliminate them.
- Standard Operating Procedures (SOPs) Creation: Writing and updating clear guidelines to ensure consistency in production and quality control in the pharma industry.
- Training: Teaching their employees about GMP rules, SOPs, and quality expectations to keep everything on track.
- Deviation Handling: Looking into any process deviations and making improvements to stop them from happening ever again.
How QA Ensures Compliance with GMP
To meet Good Manufacturing Practice (GMP) standards, Quality Assurance (QA) works to prevent quality problems before they even happen. This means conducting regular audits, keeping an eye on processes in real-time, and making sure everyone in the pharmaceutical industry values quality. By spotting risks early and fixing them, QA helps keep manufacturing on track, ensures compliance with regulations, and avoids expensive issues like product recalls.
What is Quality Control (QC)?
Quality Control (QC) is a reactive, product-focused process designed to verify that pharmaceutical products meet established quality standards before they reach the market. Unlike QA, which builds quality into the process, QC focuses on detecting and addressing defects through rigorous testing and inspections at various stages of production. Effective QC ensures that only safe, effective, and compliant products are distributed, minimizing regulatory risks and safeguarding patient health.
Core Responsibilities of QC
Quality Control is essential for making sure pharmaceutical products are safe, effective, and meet regulatory standards. It focuses on thorough testing, monitoring, and validation at different stages to catch and fix any quality issues before a product is released.
- Analytical Testing: Running lab tests to check the chemical makeup, strength, and purity of drug formulations.
- Stability Studies: Assessing how well a product maintains its effectiveness and shelf life under different environmental conditions.
- Process Controls: Monitoring key production parameters to ensure that every batch adheres to quality control in the pharma industry specifications throughout the manufacturing process.
- Final Product Release: Ensuring that only fully compliant products meeting all GMP and regulatory requirements are approved for market distribution.
QC Techniques in the Pharmaceutical Industry
Modern QC methods integrate advanced analytical techniques to maintain product consistency and regulatory compliance. Some of the most widely used QC techniques include:
- High-Performance Liquid Chromatography (HPLC): Essential for identifying and quantifying active pharmaceutical ingredients (APIs).
- Fourier Transform Infrared Spectroscopy (FTIR): Used for material verification and detecting impurities.
- Sterility Testing: Ensures products are free from microbial contamination, which is crucial for injectables and biologics.
- Dissolution Testing: Verifies the release rate and bioavailability of active ingredients to ensure consistent therapeutic effectiveness.
A well-integrated QA and QC strategy is essential for pharmaceutical companies to maintain high-quality standards, avoid compliance issues, and ensure patient safety while optimizing manufacturing efficiency.
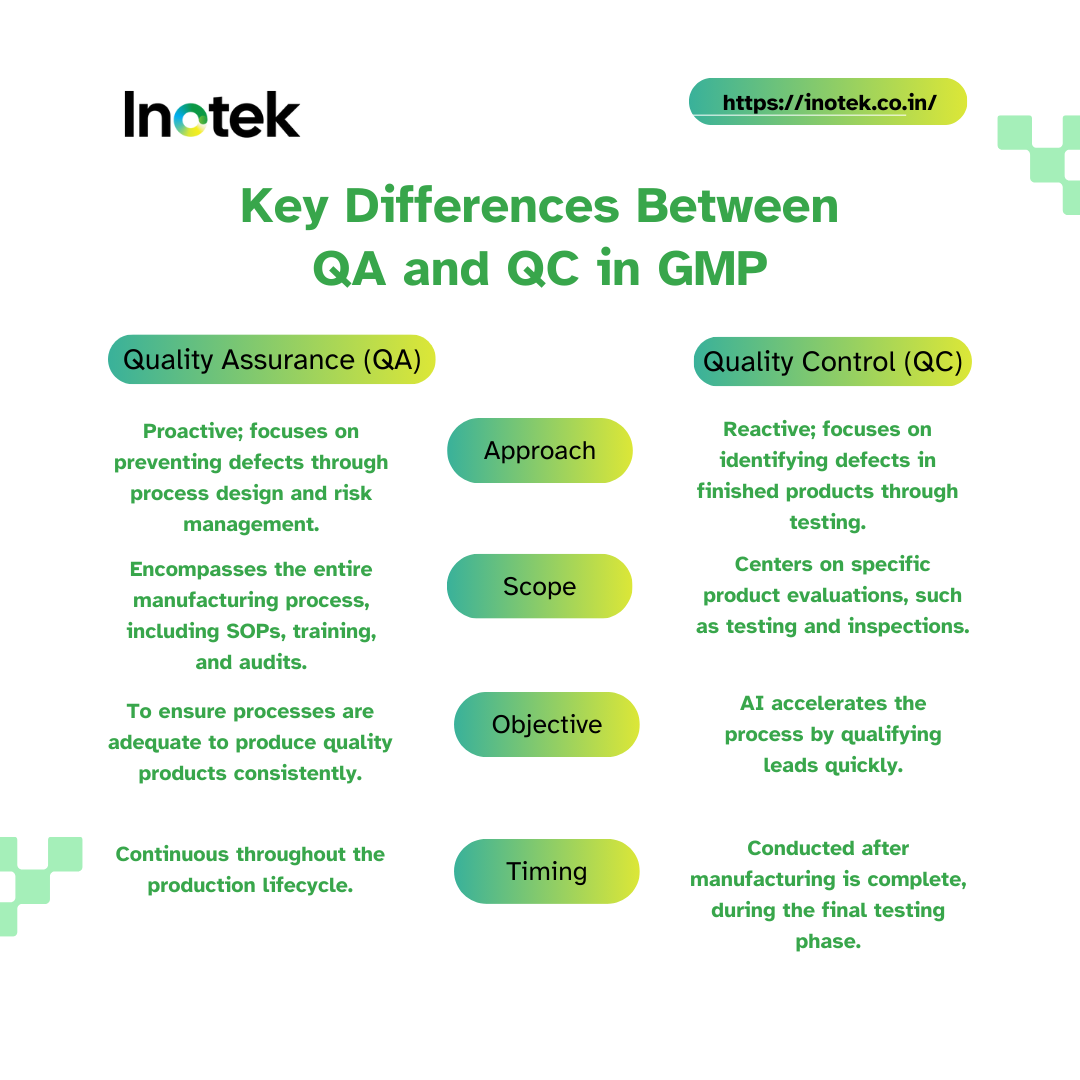
Key Differences Between QA and QC in GMP
Understanding the difference between Quality Assurance (QA) and Quality Control (QC) is essential for effective quality management. While both aim to ensure high product quality, they focus on different aspects of the process and require distinct approaches.
Why Both QA and QC Are Essential in GMP
Both Quality Assurance and Quality Control play a key role in making sure pharmaceutical products are safe, effective, and high-quality. QA focuses on setting up strong processes to prevent mistakes, while QC acts as a checkpoint to catch and fix any issues that slip through. Working together, they build a solid quality system that ensures every product meets the required standards and is safe to use.
Regulatory Requirements for QA and QC
Compliance with international regulations is mandatory in the pharmaceutical industry. Key guidelines include:
- World Health Organization (WHO) GMP: Provides global standards for GMP quality assurance in pharmaceutical manufacturing.
- U.S. Food and Drug Administration (FDA) CFR 210/211: Outlines current GMP regulations for manufacturing, processing, packing, or holding drugs.
- European Union (EU) GMP Part I & II: Details GMP requirements for medicinal products and active substances within the EU.
Consequences of Neglecting QA or QC
Ignoring Quality Assurance and Quality Control in pharmaceutical manufacturing can have serious consequences, from legal trouble to patient safety risks.
- Product Recalls: Skipping GMP standards can lead to massive recalls, shaking consumer trust and disrupting business.
- Legal and Regulatory Penalties: Not meeting compliance rules can result in hefty fines, operational shutdowns, or restrictions.
- Reputational Damage: Poor quality control can ruin a company's reputation, making it tough to regain market credibility.
- Supply Chain Disruptions: Weak quality management can cause delays, shortages, and higher production costs.
- Compromised Patient Safety: Low-quality drugs can pose serious health risks, putting lives in danger.
Challenges in Implementing QA and QC in Pharma
Implementing QA and QC processes is not without their challenges. Common obstacles for companies with quality control issues, include data integrity issues, process deviations, and ensuring consistent regulatory compliance. Addressing these challenges requires a commitment to continuous improvement and adaptation to evolving industry standards.
Common QA Challenges in GMP Facilities
A strong QA framework is essential for consistency in pharmaceutical manufacturing, but several obstacles can compromise quality and compliance:
Documentation Errors
- Inaccurate, incomplete, or outdated records can result in compliance failures and impact product quality.
- Digital documentation systems and real-time audits can mitigate human errors.
Standard Operating Procedure (SOP) Deviations
- Failure to adhere to established protocols leads to inconsistencies and defects.
- Regular training and automated SOP enforcement tools can improve adherence.
Risk Management Gaps
- Weak identification and mitigation of risks may cause unforeseen quality issues.
- Proactive risk assessment models and AI-driven predictive analytics enhance risk management.
Common QC Challenges in Pharmaceutical Testing
Ensuring product safety, efficacy, and regulatory compliance requires robust QC measures. However, several issues can compromise testing reliability:
Testing Failures
- Equipment malfunctions and human errors can lead to inaccurate results.
- Implementing stringent validation protocols and automated data capture reduces errors.
Lab Equipment Calibration Issues
- Uncalibrated or poorly maintained equipment produces unreliable data.
- Regular maintenance schedules and AI-driven monitoring improve calibration accuracy.
Inaccurate Batch Testing
- Errors in sampling or testing protocols can misrepresent product quality.
- Advanced real-time analytics and automated batch tracking enhance accuracy.
How to Improve QA and QC Systems in Pharma
Enhancing QA and QC systems involves adopting best practices and leveraging technological advancements. Key strategies include:
Best Practices for Strengthening QA
QA is a proactive methodology aimed at preventing errors before they occur. Strengthening QA involves embedding systematic processes that improve product reliability and regulatory compliance.
- Risk-Based Auditing: Conducting scheduled audits to identify and address potential quality risks before they escalate. Early detection helps minimize disruptions and ensures adherence to GMP guidelines.
- Automation and Digitalization: Implementing automated quality management systems (QMS) to enhance traceability, documentation, and reporting. Digital tools help streamline workflows and minimize human errors.
- Comprehensive Documentation: Ensuring real-time, accurate record-keeping to meet compliance requirements and support efficient decision-making during regulatory inspections.
Best Practices for Enhancing QC Processes
QC is the final checkpoint before pharmaceutical products reach the market, ensuring that only safe and effective products are distributed. Strengthening QC requires robust testing procedures, real-time monitoring, and strict adherence to industry standards.
- Real-Time Monitoring: Utilizing data-driven approaches to track production parameters and immediately identify deviations. This enables quick intervention and reduces the likelihood of non-compliance.
- Advanced Analytical Testing: Leveraging high-precision testing methods such as High-Performance Liquid Chblogromatography (HPLC) and sterility testing to verify the consistency and purity of pharmaceutical products.
- Automated Reporting: Reducing manual documentation errors through digital reporting tools, which ensure accuracy, enhance compliance, and streamline regulatory submissions.
Transitioning from Quality Control to Quality Assurance
Shifting from a QC-driven model to a QA-focused approach can help pharmaceutical companies achieve better compliance and efficiency. The transition involves integrating proactive strategies that ensure quality is built into every stage of production rather than merely inspecting final outputs.
Why the Shift is Necessary in Modern Pharma
The pharmaceutical industry is changing, and relying only on QC to catch mistakes isn’t enough. Shifting to a proactive QA strategy helps prevent issues before they happen, ensuring better efficiency, stronger compliance, and consistent product quality in the long run.
- Proactive vs. Reactive: Preventing defects is more efficient and cost-effective than fixing them post-production. By integrating QA processes early, companies can avoid costly recalls and production delays.
- Regulatory Expectations: Global regulatory bodies, including the FDA and EMA, emphasize risk-based approaches, making a quality control to quality assurance transition preferable. Implementing QA from the start ensures better alignment with evolving regulatory standards.
- Enhanced Product Safety and Efficacy: QA ensures that pharmaceutical products meet safety, potency, and purity standards throughout the manufacturing lifecycle.
Steps to Transition from QC to QA Approach
Shifting to a QA-driven system takes more than just policy changes—it requires smart use of technology, continuous process improvements, and a strong quality-first mindset across all levels of the organization.
- Adopting Continuous Improvement Programs: Implementing process improvements based on data analysis and risk assessments to refine workflows and enhance quality output.
- Leveraging AI and Automation: Using AI-driven quality monitoring, predictive analytics, and automated data collection to proactively identify and mitigate quality deviations.
- Embedding a Quality-First Mindset: Ensuring all team members—from production floor workers to executives—prioritize quality throughout every phase of manufacturing.
- Strengthening Risk-Based Quality Management: Enhancing raw material screening processes, aligning with evolving pharmacopoeia standards, and integrating advanced risk assessment methodologies.
Setting the Standard: Inotek’s Approach to Quality Assurance & QC
In an industry where precision, compliance, and safety define success, Inotek sets the standard with a seamless integration of Quality Assurance and Quality Control. Our expertise in GMP quality compliant systems, risk-based auditing, and AI-driven monitoring ensures that pharmaceutical companies and pharma-construction project management firms achieve uncompromising quality, regulatory confidence, and operational efficiency.
If you want to simplify quality processes, stay on top of compliance rules, and keep your pharmaceutical operations future-ready, Inotek has your back. We’re all about helping companies with quality control issues build strong, reliable quality systems that actually work.
Let’s team up to take your QA and QC difference standards to the next level—reach out to Inotek and see what we can do for you!
FAQs
What is the difference between QA and QC in the pharma industry?
Quality Assurance (QA) is a proactive, process-focused approach that prevents defects by ensuring GMP quality compliance throughout production. Quality Control is reactive, product-focused, identifying defects through testing and inspections to ensure pharmaceutical products meet safety, efficacy, and regulatory standards before release.
Why is GMP quality important for quality control in the pharmaceutical industry?
Good Manufacturing Practice (GMP) ensures consistent quality, safety, and compliance in pharmaceutical manufacturing. It helps prevent contamination, deviations, and defects, reducing regulatory risks and product recalls. Adhering to GMP quality guidelines strengthens consumer trust and ensures that medicines meet strict health and safety standards.
How do companies transition from quality control to quality assurance?
Shifting from QC to QA requires risk-based auditing, automation, and continuous improvement to build quality into production rather than just testing final products. Companies integrate AI-driven monitoring, real-time analytics, and SOP standardization to prevent defects, ensuring regulatory compliance and operational efficiency.
What are common quality control issues in the pharma industry?
Common QC challenges include testing failures, equipment calibration errors, batch inconsistencies, and data integrity issues. Poor lab practices, deviations from SOPs, and inadequate documentation can lead to compliance failures, product recalls, and regulatory penalties, affecting patient safety and company reputation.