Publisher
India Pharma Outlook
published at
March 27, 2025
Good Manufacturing Practices (GMP): Complete Compliance & Facility Guide
Is your facility aligned with Good Manufacturing Practices (GMP), or are hidden compliance gaps putting operations at risk? In pharmaceutical and biotech industries, failing to meet GMP guidelines can lead to production halts, recalls, and regulatory penalties. This guide outlines essential aspects of GMP compliance, including facility design, workflow optimization, and regulatory readiness.
What Are Good Manufacturing Practices (GMP)?
Good Manufacturing Practices (GMP) are a set of regulations ensuring consistent product quality and safety. In the pharma industry, following GMP rules is key to making sure medicines are safe and work as they should. These guidelines cover every stage of production, from sourcing raw materials and manufacturing to quality checks and distribution, ensuring consistently high standards.
Key Principles of GMP Compliance
Achieving GMP compliance involves adhering to the following fundamental principles:
- Quality Management: Implementing a quality management system (QMS) for production and control.
- Personnel: Ensure proper training and maintain high hygiene standards.
- Facilities & Equipment: Keep infrastructure contamination-free and use validated equipment as per GMP guidelines.
- Documentation: Maintain detailed records for all processes.
- Production: Follow standardized procedures for consistency.
- Quality Control: Regularly test products to ensure quality.
- Self-Inspection: Perform internal audits for compliance verification.
GMP Quality Management Systems
A robust quality management system (QMS) is the cornerstone of GMP compliance. It encompasses all activities that determine quality policies, objectives, and responsibilities. According to the World Health Organization (WHO), GMP defines quality measures for both production and quality control, ensuring that processes are clearly defined, validated, reviewed, and documented. This includes establishing strong quality management systems, obtaining appropriate quality raw materials, and maintaining reliable testing laboratories.
GMP Personnel Training & Hygiene
Manufacturing staff should regularly train on GMP guidelines relevant to their jobs. This helps them understand the importance of hygiene and following proper procedures. Frequent training sessions and assessments keep employees up to date with the latest GMP standards and best practices.
GMP Facility & Equipment Standards
Facilities and equipment should be well-designed and maintained to prevent contamination and allow for easy cleaning. Regular calibration and validation of equipment help ensure accuracy and reliability in production. Adhering to CGMP regulations is crucial for ensuring drug products meet the required standards for identity, strength, quality, and purity through strict oversight of manufacturing processes.
GMP and It's Importance in Manufacturing
GMP plays a very important role in maintaining product quality and safety and securing regulatory approval. By following GMP guidelines, manufacturers can minimize risks such as contamination, mix-ups, and errors, ensuring that products are consistently produced to the required quality standards. This not only protects consumers but also enhances the company's reputation and compliance with regulatory authorities.
Core Principles of GMP Compliance
The five main principles of GMP compliance include:
- Hygiene: Prevent contamination with strict cleaning standards.
- Documentation: Maintain detailed records.
- Quality Control: Conduct regular inspections.
- Validation: Ensure consistency in production.
- Training: Regular employee education on GMP compliance.
Why GMP Compliance is Crucial for Pharmaceuticals and Biotech?
For pharmaceutical and biotech companies, GMP compliance is not just a regulatory requirement but a critical component of operational excellence. Compliance ensures that products are safe for consumer use and meet the efficacy standards claimed. Regulatory bodies such as the FDA, WHO, and EMA require strict adherence to GMP guidelines for product approval and market authorization. Non-compliance can lead to severe consequences, including product recalls, legal actions, and damage to the company's reputation.
GMP Guidelines for Pharmaceutical Manufacturing
Pharmaceutical manufacturing under GMP guidelines follows very strict controls and standardized procedures in order to maintain product quality and safety. These guidelines help in reducing risks in production that testing alone cannot eliminate.
Facility Layout & Infrastructure
Designing a GMP-compliant facility requires careful planning to prevent cross-contamination and maintain an efficient workflow for materials and personnel.
- Cleanroom Design: Controlled environments with defined cleanliness levels.
- HVAC Systems: Proper ventilation, air filtration, and pressure control.
- Contamination Control: Segregation of materials and personnel.
Environmental & Equipment Standards
- Air Quality: HEPA filters maintain clean air.
- Temperature & Humidity Control: Ensures product stability.
- Sanitization: Routine cleaning and disinfection of surfaces and equipment.
Personnel Training & Hygiene Standards
Employees must be trained in GMP principles and specific procedures relevant to their respective roles. This training should cover proper hygiene practices, including the use of protective clothing, handwashing techniques, and protocols to prevent contamination. Regular assessments and refresher courses help maintain high standards of compliance and awareness.
Steps to Achieve GMP Compliance
Achieving GMP compliance involves a systematic approach:
- Gap Analysis: Identify areas needing improvement.
- Develop SOPs: Document standardized processes aligned with GMP guidelines.
- Train Personnel: Regular training programs.
- Implement Quality Control: Frequent product testing.
- Maintain Documentation: Ensure compliance records are up-to-date.
- Regulatory Readiness: Conduct internal audits.
GMP Documentation & Record-Keeping
Keeping accurate records is essential for GMP compliance. Important documents include:
- Batch Records: Track production for traceability.
- Standard Operating Procedures (SOPs): Outline production steps.
- Validation Reports: Verify process consistency.
- Deviation Reports: Record and address any issues.
A well-organized documentation system not only ensures compliance but also helps identify areas for improvement in manufacturing processes.
GMP Audits & Inspections: What to Expect
Regulatory audits are conducted by agencies like the FDA, WHO, and EMA to verify GMP compliance. Key aspects of an inspection include:
- Facility & Equipment Conditions
- Personnel Training & SOP Adherence
- Quality Control & Documentation
Proper preparation, including conducting internal audits and addressing potential non-compliance areas, helps companies pass inspections successfully.
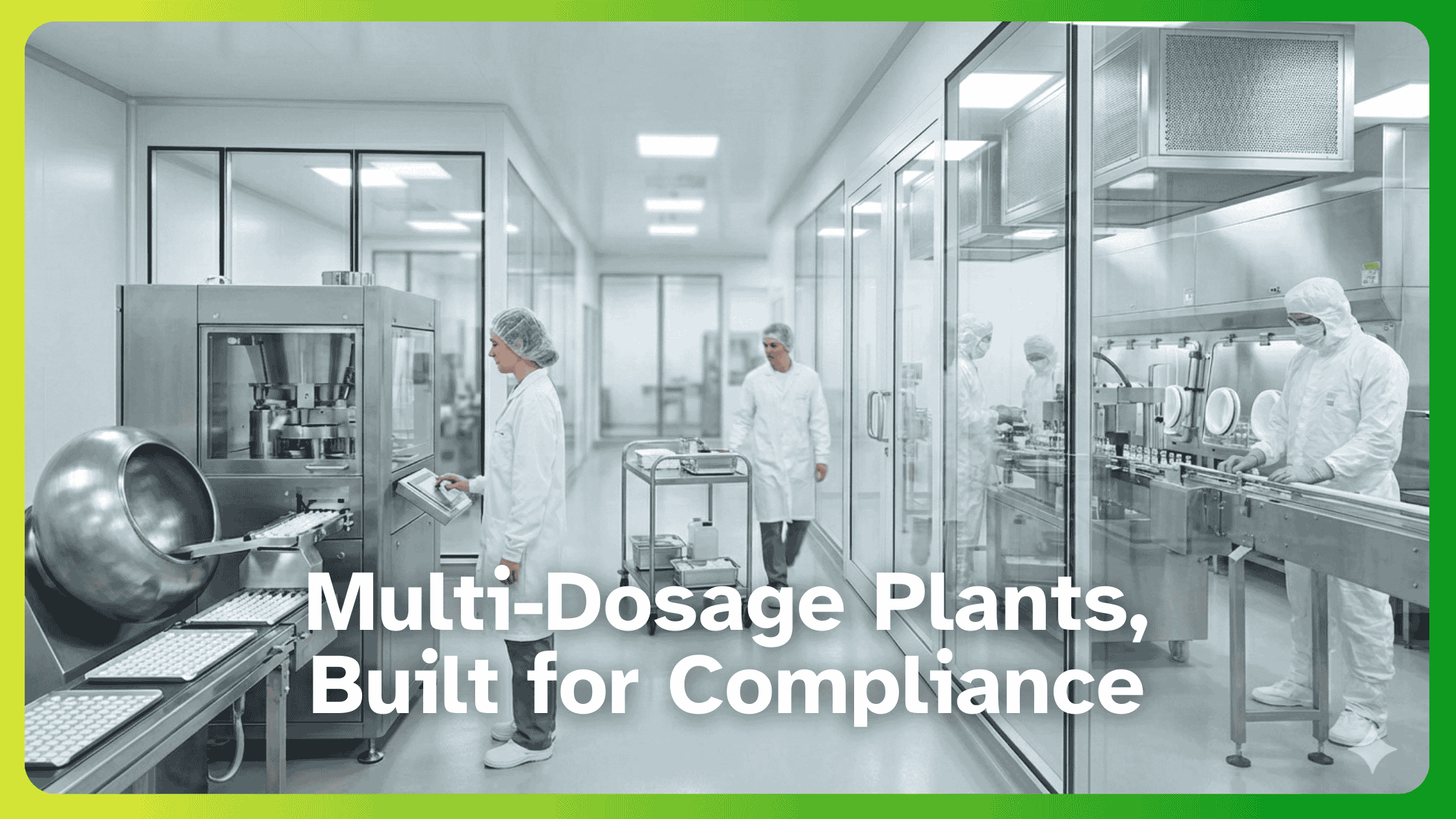
GMP Facility Design & Infrastructure Best Practices
A GMP-compliant facility must be designed to prevent contamination, ensure efficiency, and meet global regulatory standards like FDA, WHO, and EMA GMP guidelines. Proper layout enhances compliance, reduces risks, and ensures smooth regulatory approval.
Key design elements:
- Logical Workflow: Minimize the contamination risks.
- ISO-Classified Cleanrooms: Maintain air purity and pressure controls.
- Easy-to-Clean Materials: Prevent the microbial growth.
- Contamination Control Zones: Gowning areas and restricted access zones.
Inotek ensures GMP-compliant facility design with cost-effective, regulatory-approved solutions.
GMP-Compliant Facility Layout & Workflow
- Logical Material Flow: Prevent cross-contamination.
- Dedicated Storage Areas: Separate raw materials, intermediates, and finished products.
- Ventilation & Airflow: Maintain controlled environments per GMP facility guidelines.
Environmental & Cleanroom Standards in GMP
Air Quality: Use high-efficiency particulate air (HEPA) filters to maintain clean air standards.
Temperature & Humidity Control: Maintain optimal conditions to prevent product degradation.
Sanitization & Contamination Control: Implement strict cleaning schedules to maintain hygiene.
Sanitation & Hygiene Protocols for GMP Compliance
- Scheduled Cleaning & Disinfection
- Protective Clothing for Employees
- Hand Hygiene & Sanitization Stations
Regulatory Bodies & GMP Certification Process
Achieving GMP certification requires compliance with regulations set by various global agencies, including:
- U.S. Food and Drug Administration (FDA)
- World Health Organization (WHO)
- European Medicines Agency (EMA)
- Medicines and Healthcare products Regulatory Agency (MHRA)
The certification process involves application submission, facility inspection, and compliance verification before approval.
Turnkey Solutions for GMP Compliance – How Inotek Helps
Inotek specializes in delivering GMP-compliant facilities for pharmaceutical and biotech companies, ensuring full adherence to FDA, WHO, EMA, and MHRA regulations. With deep expertise in GMP facility guidelines, construction, validation, and regulatory compliance, Inotek simplifies GMP implementation, minimizing compliance risks and project delays.
End-to-End Facility Design & Construction
Inotek offers turnkey solutions for GMP-compliant manufacturing facilities, including:
- Regulatory-Aligned Facility Layouts – Optimized workflows to prevent cross-contamination.
- Validated Equipment & Utility Systems – HVAC, water purification, and air handling for GMP compliance.
- Scalable & Customizable Designs – For pharma production, R&D, and aseptic processing.
- Integration of Digital Compliance Tools – Automated monitoring and electronic batch records.
Regulatory Compliance & Inspection Readiness
Inotek ensures facilities are audit-ready and meet global GMP guidelines by offering:
- Pre-Inspection Audits & Risk Assessments – Identifying compliance gaps before regulatory reviews.
- Documentation & Validation Support – Aligning SOPs, batch records, and equipment validation with FDA & WHO guidelines.
- Regulatory Training & Corrective Action Plans – Preparing teams for inspections and post-audit compliance strategies.
Partner with Inotek for seamless GMP facility design, construction, and regulatory compliance solutions.
Common Challenges in GMP Implementation
GMP compliance comes with challenges in facility design, documentation, equipment validation, and staff training, all of which affect regulatory approval and operational efficiency.
- Facility Design Challenges – Limited space and poorly planned cleanrooms can compromise contamination control, airflow, and material segregation, leading to compliance risks.
- Documentation Gaps – Incomplete or outdated records and failure to follow SOPs can result in regulatory violations and inconsistencies in production.
- Equipment Validation Issues – Regular calibration and maintenance is an essential, but unexpected breakdowns and delays can always disrupt operations.
- Staff Training Hurdles – Inconsistent training may create knowledge gaps, leading to errors that impact process control and product quality.
- Facility Design & Compliance Gaps – Inefficient facility layouts, messy documentation, and validation issues can increase the chances of failing inspections and running into regulatory trouble.
How Inotek Helps: Inotek specializes in facility design, documentation, and validation, ensuring GMP compliance, minimizing regulatory risks, and enhancing operational efficiency.
Why Choose Inotek for GMP-Compliant Facilities?
Achieving GMP compliance demands precise facility design, quality control, regulatory expertise, and continuous training to avoid violations and inefficiencies. Inotek offers turnkey solutions for GMP-compliant facilities, ensuring seamless implementation and adherence to FDA, WHO, EMA, and MHRA standards.
What Sets Inotek Apart?
- End-to-End Facility Solutions: From conceptual design to final construction and regulatory validation.
- Compliance-Driven Designs: Facility layouts optimized for contamination control, workflow efficiency, and GMP adherence.
- Regulatory Expertise: Support in audits, documentation, and training to ensure seamless regulatory approvals.
- Custom-Built GMP Solutions: Scalable and adaptable to meet the evolving needs of pharmaceutical and biotech industries.
Partner with Inotek to build GMP-compliant facilities that meet global regulatory standards while optimizing cost, efficiency, and long-term compliance. Get in touch today to discuss your facility requirements and take the next step toward seamless GMP compliance.
FAQs
What are the key GMP guidelines for pharmaceutical manufacturing?
Key GMP guidelines ensure strict quality control, validated processes, proper documentation, personnel training, contamination prevention, and regulatory compliance to maintain product safety and efficacy.
How does GMP compliance impact product quality and safety?
GMP compliance minimizes contamination risks, ensures consistency, and meets regulatory standards, safeguarding consumer health.
What are the critical facility requirements for a GMP-compliant facility?
A GMP facility must have clean room classifications, HVAC control, contamination prevention, validated equipment, and strict hygiene protocols.
How can Inotek help companies meet GMP compliance standards?
Inotek provides end-to-end GMP solutions, including facility design, equipment validation, regulatory training, and audit preparation to ensure seamless compliance.