Publisher
India Pharma Outlook
published at
September 3, 2025
Carbon Footprint Reduction in Pharmaceutical Supply Chains: A Compliance Perspective
Is your pharmaceutical supply chain built to meet tomorrow’s climate regulations without risking today’s compliance?
With climate regulations tightening and sustainability expectations growing, the pharmaceutical industry is under increasing pressure to reduce its carbon footprint. This is particularly true for Scope 3 emissions, including those from packaging, logistics, and supplier operations. The burning question is: How can we reduce emissions while maintaining GMP compliance and audit readiness?
The challenge is real. Healthcare accounts for nearly 5% of global greenhouse gas emissions, with the pharmaceutical sector playing a significant role. From high-energy API manufacturing to cold-chain transport and disposable packaging, the typical pharmaceutical supply chain presents multiple carbon hotspots, all of which must be addressed without disrupting validation protocols or patient safety standards.
This article outlines a structured roadmap for reducing the carbon footprint in pharmaceutical supply chains without compromising on compliance. You’ll learn:
- Where emissions are most concentrated across the pharma lifecycle
- How global leaders like AstraZeneca, GSK, and Novartis are responding
- What GMP-aligned interventions can be applied across manufacturing, logistics, and vendor engagement
- How engineering, QA, and project teams can build sustainable, inspection-ready systems
Whether you're part of a pharmaceutical operations team, a compliance lead, or a pharmaceutical construction project manager supporting facility builds, this guide will help you navigate the evolving expectations around sustainability and ensure your infrastructure is both climate-conscious and regulation-compliant.
Where Emissions Occur in the Pharmaceutical Supply Chain
Carbon emissions in pharmaceutical supply chains occur across the entire product lifecycle, from raw material extraction to final delivery. Major hotspots include energy-intensive manufacturing, cold-chain logistics, and packaging waste. These stages are not only emission-heavy but also subject to strict regulatory and Good Manufacturing Practice (GMP) standards, making optimisation complex but essential.
API Manufacturing and Energy Use
API (Active Pharmaceutical Ingredient) manufacturing is one of the most resource-intensive steps in the supply chain. According to ScienceDirect, this stage demands significant heat, power, and water usage, particularly in chemical synthesis and solvent recovery processes. The use of organic solvents, many of which are hazardous and energy-intensive to handle, adds further environmental strain.
Cleanroom environments compound the issue. These spaces require the continuous operation of HVAC systems to maintain ISO-class air quality, which can consume up to 65% of the total facility's energy alone. The combination of high solvent use and strict air handling demands makes API production a critical focus for emission reduction.
For pharmaceutical construction companies, this means that HVAC zoning, energy modelling, and process intensification must be integrated early in facility planning to curb emissions without compromising Good Manufacturing Practice (GMP) compliance.
Watch how hidden energy drains inside pharma plants create both compliance risks and carbon challenges — and why re-engineering cleanrooms, utilities, and equipment is the way forward.
Logistics and Cold Chain Transport Emissions
Pharma logistics, especially cold chain distribution is another major contributor to carbon emissions. As highlighted by Pharmaceutical Technology, temperature-sensitive biologics and vaccines must be kept within strict temperature ranges (2–8°C or even lower), often across long distances. This requires energy-hungry refrigeration units, foam-insulated packaging, and dry ice, all of which elevate the supply chain's carbon load.
Air freight, frequently used due to the high value and shelf-life limitations of many pharma products, has a much higher carbon footprint than land or sea transport. Fuel dependency, route inefficiencies, and redundant packaging exacerbate the impact.
For pharmaceutical stakeholders, optimizing cold chain logistics involves transitioning to electric delivery fleets, implementing route optimization technologies, and utilizing phase-change materials in packaging. Construction partners must support these needs with appropriate dock design, cold room insulation, and automated monitoring systems.
Single-Use Packaging and Disposal Challenges
The pharmaceutical industry relies heavily on single-use plastics for sterility and regulatory compliance. From blister packs to shrink wraps and sterile vials, these materials are often non-recyclable due to contamination or mixed-material composition, according to Pharmaceutical Technology. The environmental impact is twofold: the carbon-intensive manufacturing of plastics and the post-use incineration or landfill disposal of plastics.
Moreover, the increasing adoption of high-potency APIs (HPAPIs) has led to the greater use of disposable containment systems, as they simplify cleaning validation. However, this shift has also raised the volume of biomedical and hazardous waste across the supply chain.
For engineering and QA teams in pharmaceutical companies, this presents a need to strike a balance between compliance, safety, and sustainability. This challenge can be addressed through early packaging design reviews, lifecycle assessments, and collaboration with waste management vendors.
Solutions for Each Hotspot
Despite the challenges, practical and compliant solutions exist:
- Hybrid or electric delivery fleets can reduce emissions in transport-intensive segments.
- Smart HVAC systems, integrated with variable-speed drives and energy recovery, can significantly reduce power consumption in cleanrooms.
- Digital twins and simulation models help optimise utility layouts and predict carbon output during the early design phase.
- Process intensification and closed-loop solvent systems reduce energy and material waste in API manufacturing.
- Biodegradable or mono-material packaging solutions enable better recyclability while meeting Good Manufacturing Practice (GMP) standards.
Leading biopharma companies are already applying these methods to achieve meaningful carbon reduction. Embedding these solutions during the planning and execution phases ensures that pharma facilities are built not only for today’s compliance but also for tomorrow’s climate goals.
Aligning Carbon Reduction with Global Compliance Standards
Sustainability in the pharmaceutical industry is no longer a siloed CSR initiative; it must be embedded into operations and validated processes. However, reducing the carbon footprint across the pharmaceutical supply chain must be done in a way that doesn’t compromise regulatory obligations under GMP, FDA, EMA, or country-specific standards.
This is especially important for pharma stakeholders responsible for quality, engineering, and supply chain, along with project partners like Inotek, who must integrate sustainable practices into facility design, HVAC planning, cold chain operations, and vendor compliance systems.
The way forward involves a compliance-first approach to sustainability. That means deploying climate-conscious upgrades that still pass inspections, maintain product safety, and meet documentation and validation standards.
GHG Protocol and Scope 1–3 Emissions in Pharma
The Greenhouse Gas (GHG) Protocol classifies emissions into three scopes:
- Scope 1: Direct emissions from owned or controlled sources, like on-site boilers or company vehicles
- Scope 2: Indirect emissions from purchased electricity or steam
- Scope 3: All other indirect emissions across the value chain, including supplier emissions, business travel, logistics, and product use
As noted by IQVIA, Scope 3 emissions account for over 70% of the total carbon footprint reduction in pharmaceutical companies, but they are also the most challenging to measure and control. This makes supply chain partners a major focus area for emission reduction efforts.
For internal teams, the priority is to adopt standardized tools for tracking all three scopes and ensure any emissions reporting aligns with ESG frameworks. For pharmaceutical construction firms, this means designing infrastructure that supports Scope 1 and 2 reductions (e.g., solar integration, smart HVAC) while enabling better supplier-level monitoring and collaboration for Scope 3.
EU Green Deal and Carbon Border Adjustments
The EU Green Deal introduces sweeping changes to how imported goods, including pharmaceuticals, will be taxed and evaluated based on their carbon intensity. One key mechanism is the Carbon Border Adjustment Mechanism (CBAM), which acts as a climate tariff for high-carbon imports into the EU.
Pharma exporters that can’t demonstrate carbon-efficient operations or full emissions disclosure across their supply chains may face penalties or trade limitations under these regulations, as outlined in Pharmaceutical Technology’s coverage of COP26 developments.
To remain competitive, pharma companies operating in or exporting to Europe must:
- Embed emission tracking and reporting across product lifecycles
- Decarbonise their facilities and transport chains.
- Evaluate suppliers against the EU-aligned ESG standards
Project partners can play a key role by advising clients early in project planning on material selection, energy modelling, and packaging optimisation that aligns with EU climate expectations.
FDA, EMA, and GMP Considerations
Any sustainability initiative must be carefully reviewed to ensure GMP compliance is not compromised. The Pharmaceutical Technology team emphasises that retrofitting equipment or switching materials even for environmental benefit can lead to regulatory pushback if not properly validated.
Key concerns include:
- Maintaining validated state: New HVAC systems or process changes must go through full qualification (DQ, IQ, OQ, PQ)
- Documentation requirements: ESG-driven changes must be traceable and documented in line with GMP
- Risk to product quality: Material substitutions (e.g., sustainable packaging) must undergo risk assessments to ensure sterility, barrier protection, and patient safety
This is where pharmaceutical construction project firms are critical. They must ensure that any proposed sustainability upgrade during plant design, commissioning, or retrofitting complies with regional and global Good Manufacturing Practice (GMP) mandates. Integration of sustainable design and GMP-compliant validation practices from the outset is the only viable path forward.
Engaging Suppliers to Build a Greener Pharmaceutical Supply Chain
A significant share of pharmaceutical supply chain emissions lies outside the company’s direct control, particularly with vendors and raw material suppliers. These Scope 3 emissions encompass energy use by suppliers, packaging manufacturing, waste treatment vendors, third-party logistics, and other related activities. Tackling these emissions is impossible without robust supplier engagement.
As Pharmaceutical Technology notes, tackling these emissions is impossible without the engagement of suppliers. Pharma companies must shift from transactional relationships to strategic collaborations that prioritise sustainability alongside quality and compliance.
For internal teams, this means integrating ESG into procurement strategy. For pharma-construction partners, it’s about helping clients build compliant infrastructure and workflows that enable greener supplier integration, from vendor assessment templates to logistics zone planning.
Setting ESG Criteria for Suppliers
Pharma firms are increasingly using formalised ESG (Environmental, Social, Governance) criteria to qualify and monitor their suppliers. These standards go beyond price and delivery metrics; they include expectations for:
- Carbon reporting
- Use of renewable energy
- Water conservation and chemical handling
- Labour rights and diversity
A key enabler of this is the Pharmaceutical Supply Chain Initiative (PSCI), a collective that provides a shared supplier code of conduct, tools for ethical sourcing, and ESG self-assessment protocols specifically tailored to the pharmaceutical industry.
By embedding PSCI-aligned ESG clauses into contracts, companies can ensure consistent sustainability performance across the vendor base. For project firms, designing digital ESG dashboards and audit-friendly vendor onboarding systems can help clients streamline this transition.
Joint Innovation and Best Practice Sharing
Reducing supply chain emissions requires more than individual improvements; it demands shared innovation. Many pharma leaders now co-invest in pilot projects with suppliers to trial:
- Low-emission solvents or APIs
- Electrified logistics routes
- Renewable-powered contract manufacturing sites
Additionally, shared carbon accounting tools and centralised sustainability data platforms enable both pharmaceutical companies and suppliers to track and reduce emissions collaboratively.
As Pharmaceutical Technology reports, these partnerships have already led to tangible reductions, particularly in biologics cold chains and packaging redesign efforts. Pharma-construction teams play a strategic role in enabling these pilots by designing flexible utilities and validation-ready environments that accommodate new, sustainable materials and equipment.
Auditing and Incentivising Compliance
Once ESG criteria are established, enforcement is key. This is where risk-based supplier audits and third-party certifications play a crucial role. Leading pharma companies are using:
- ISO 14001 (environmental management)
- SEDEX or EcoVadis ratings
- PSCI audit frameworks
These programs help pharma firms identify high-impact suppliers, track progress, and manage risk. However, ESG shouldn’t only be policed; it must also be incentivized.
Pharma firms are increasingly offering preferred vendor status, longer contracts, or co-branding opportunities to suppliers who meet or exceed ESG targets. Construction and project management teams can support this by integrating supplier evaluation systems and ESG flagging features into digital commissioning, procurement, and qualification platforms.
Roadmap to Reduction of Carbon Footprint in Pharma Supply Chains
Building a sustainable pharmaceutical supply chain isn’t a one-time initiative; it requires a structured roadmap backed by metrics, technology, and GMP-aligned implementation. According to IQVIA, many pharma companies are now creating phased decarbonization plans that begin with measurement and proceed through interventions and continuous monitoring.
For internal pharma teams, this roadmap ensures regulatory and operational alignment. For companies, it presents a clear opportunity to embed carbon-aware choices during facility planning, system design, and vendor onboarding.
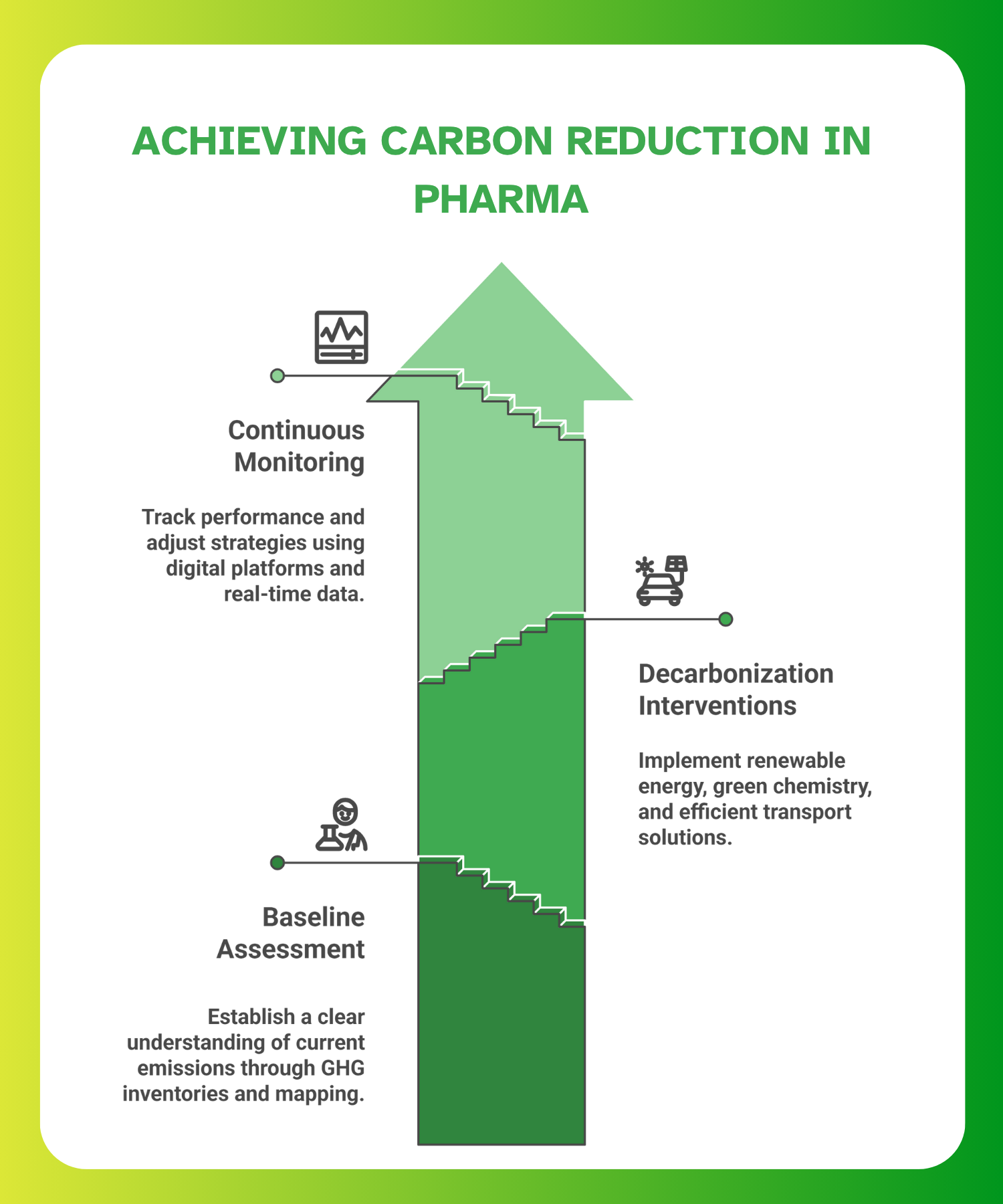
Measurement and Baseline Assessment
The first step in any carbon reduction strategy is understanding your emissions profile. IQVIA highlights that without a clear baseline across Scope 1, 2, and 3 emissions, companies risk investing in the wrong areas or underreporting to regulators.
Essential actions include:
- Conducting full GHG inventories using established protocols
- Mapping emissions per facility, product line, and distribution channel
- Using carbon accounting software that integrates with ERP and quality systems
This allows pharma teams to prioritise high-impact areas, such as HVAC energy usage, solvent recovery systems, or long-haul logistics. For project partners, this means integrating sensors, metering infrastructure, and emissions data dashboards into facility design and commissioning documentation.
Decarbonization Interventions
Once the baseline is set, pharma companies can begin implementing proven interventions. The leaders in the biopharma sector are already deploying:
- Renewable energy sources: On-site solar, green electricity purchase agreements
- Green chemistry alternatives: Low-emission solvents and process catalysts
- Low-emission transport: Switching to electric or hybrid vehicle fleets
- Facility upgrades: Smart HVAC, efficient cleanroom layouts, and process intensification
Each of these actions must be evaluated for compliance with Good Manufacturing Practice (GMP), validation protocols, and safety standards. For Pharma firms, the role becomes critical here, ensuring the decarbonization efforts do not disrupt regulated processes and that technical documentation supports both inspection and sustainability goals.
Monitoring, Reporting, and Continuous Improvement
Sustainability isn’t static. After initial interventions, pharmaceutical organisations must continuously track performance and adjust based on results and compliance feedback. IQVIA recommends using digital platforms that offer:
- Real-time carbon monitoring dashboards
- Automated ESG reporting templates
- Predictive tools to simulate energy or emission impacts before making operational changes
These systems not only support transparency for investors and regulators but also enable internal teams to detect anomalies early, such as unexpected energy spikes or transportation inefficiencies.
For pharmaceutical construction firms, it’s essential to support this layer with pre-integrated Building Management Systems (BMS), digital twins, and clear handover documentation that connects infrastructure to emission reduction goals.
Case Example: A Global Pharma Leader’s Net-Zero Journey
Several pharmaceutical leaders are setting benchmarks for carbon reduction through structured, supply chain-driven sustainability programs. Their strategies demonstrate how operational changes, when guided by data, collaboration, and compliance, can deliver a measurable impact without compromising Good Manufacturing Practice (GMP) or regulatory obligations.
These examples provide valuable guidance for teams involved in pharmaceutical manufacturing, engineering, procurement, and facility planning.
AstraZeneca’s Path to Net-Zero by 2045
AstraZeneca has committed to achieving net-zero emissions across its value chain by 2045, with a target to reduce Scope 3 emissions by 50% by 2030. According to TIME and Pharmaceutical Technology, their approach is centred on supplier engagement, manufacturing energy transition, and logistics redesign.
Key initiatives include:
- Transition to 100% renewable energy across all manufacturing operations
- Phasing in electric and hybrid vehicles for transport and distribution
- Rolling out decarbonization frameworks to top-tier suppliers, including emissions tracking and reporting protocols
- Embedding sustainability metrics into supplier contracts and operational reviews
AstraZeneca’s efforts highlight the importance of aligning facility design, process flow, and supplier systems to support carbon reduction across both upstream and downstream operations.
GSK’s Carbon Neutral Laboratory & Green Chemistry Strategy
GSK’s sustainability efforts are visible in both its infrastructure and supply chain strategy. Its R&D facility in Stevenage, UK, is LEED-certified, using energy-efficient HVAC, water systems, and lighting, as detailed in the Financial Times.
On the operational side, GSK has:
- Adopted low-impact solvents and integrated green chemistry principles in its formulation processes
- Enhanced solvent recovery systems to minimise waste and emissions
- Collaborated with suppliers through the Pharmaceutical Supply Chain Initiative (PSCI) to standardise ESG compliance and reduce upstream emissions
These efforts demonstrate how early-stage infrastructure decisions and clear supplier expectations can work in tandem to reduce emissions without compromising Good Manufacturing Practice (GMP) standards.
Novartis: Sustainable Packaging and Cold Chain Optimisation
Novartis has focused on two of the most emission-intensive areas in the pharma supply chain: packaging and cold chain logistics. As highlighted by IQVIA, their targeted interventions include:
- Developing eco-friendly packaging solutions that reduce plastic use, simplify recyclability, and maintain product protection
- Deploying route optimisation technologies to reduce fuel consumption across distribution networks
- Introducing IoT-enabled cold chain monitoring systems to ensure temperature compliance while tracking emissions in real time
This dual strategy addresses carbon efficiency and product integrity simultaneously, making it highly relevant for pharma QA teams, packaging engineers, and logistics planners working under tight regulatory controls.
These real-world examples reinforce the importance of designing pharmaceutical supply chains and operational systems that are both climate-resilient and audit-ready. Emission reduction is no longer a parallel goal; it must be embedded into the technical and compliance frameworks of every project.
Final Thought: Building Climate-Ready Pharma Supply Chains Starts Now
Reduction of carbon footprint in pharmaceutical supply chains is no longer a competitive advantage; it’s a compliance necessity. From Scope 1 emissions inside the plant to Scope 3 emissions across your suppliers and logistics partners, every link in the chain must be assessed, optimised, and aligned with regulatory standards.
This article explores how industry leaders are already making measurable progress not by compromising on Good Manufacturing Practice (GMP) but by integrating carbon-conscious strategies into validated systems, packaging design, HVAC planning, and supplier management. Whether you're responsible for quality, engineering, procurement, or facility execution, the path forward is clear: climate action must be embedded early, monitored continuously, and audited without disruption.
Future-ready pharma operations aren’t just greener, they’re smarter, more efficient, and better prepared for global regulatory shifts. Now is the time to re-engineer pharmaceutical supply chains that are sustainable, compliant, and built to last.
How Inotek Helps Future-Proof Pharma Supply Chains with Carbon-Conscious Design
The complexities of decarbonising pharmaceutical supply chains demand specialised expertise and compliance-focused execution. This is where Inotek steps in as your strategic partner.
We don’t just understand carbon reporting requirements; we engineer sustainable, GMP-aligned solutions into every facility and logistics system we deliver, aligning with global regulatory standards such as the FDA, EMA, and the EU Green Deal.
Our comprehensive approach includes:
Smart HVAC & Cleanroom Engineering:
Advanced designs that reduce energy consumption in temperature-controlled environments while maintaining ISO classifications and Annex 1 compliance.
Carbon-Conscious Utility & Process Design:
Optimised utility layouts, renewable energy integration, and solvent recovery systems that lower emissions across manufacturing operations.
Sustainable Packaging & Cold Chain Solutions:
Engineering support for recyclable materials, IoT-enabled cold chain monitoring, and efficient distribution dock designs.
Digital Twin Simulations:
Emissions forecasting and scenario modelling to validate decarbonization strategies before implementation.
Audit-Ready ESG Documentation:
End-to-end traceability for all sustainability upgrades, fully integrated with DQ, IQ, OQ, and PQ validation protocols.
By partnering with Inotek, pharma manufacturers have achieved:
✅ Reduction of Scope 1 and 2 emissions across manufacturing sites
✅ Streamlined ESG reporting and compliance with the EU Green Deal
✅ Faster qualification of sustainable retrofits without disrupting operations
While compliance forms the foundation, successful decarbonization must also address growing industry expectations around the following:
🌿 Environmental sustainability and carbon disclosure
🌍 Resilient global supply chains and risk mitigation
💻 Digital compliance and real-time emissions monitoring
At Inotek, we ensure your supply chain isn’t just audit-ready but engineered for long-term operational excellence.
Recognised among the Top 10 Pharma Turnkey Contractors & Project Consultants in 2022 & 2025, Inotek helps pharma leaders design, build, and optimise supply chains and facilities that meet the strictest GMP and sustainability standards.
📞 Connect with our experts today or visit www.inotek.co.in to schedule a consultation with Mr. Rohit Ochaney.
Whether you’re planning a greenfield facility or decarbonising an existing operation, Inotek ensures your project is compliant, resilient, and future-proof.
FAQ’s
What is the carbon footprint of a pharmaceutical supply chain?
The carbon footprint of a pharmaceutical supply chain refers to the total greenhouse gas emissions generated during the manufacturing, packaging, logistics, and supplier operations of a pharmaceutical product. Scope 3 emissions such as those from raw materials and transportation often account for over 70% of total emissions.
How can pharmaceutical companies reduce their carbon footprint?
Pharmaceutical companies can reduce their carbon footprint by adopting energy-efficient HVAC systems, utilising renewable energy, optimising cold chain logistics, switching to biodegradable packaging, and establishing ESG criteria for suppliers. These changes must remain GMP-compliant and auditable.
What are Scope 1, 2, and 3 emissions in pharma supply chains?
- Scope 1: Direct emissions from owned sources (e.g., boilers, vehicles)
- Scope 2: Indirect emissions from purchased electricity or steam
- Scope 3: Indirect emissions from suppliers, logistics, packaging, and end-use
Most emissions in the pharmaceutical supply chain fall under Scope 3.
Why is carbon reduction important in pharmaceutical manufacturing?
Carbon reduction is crucial in pharmaceutical manufacturing to meet regulatory requirements, minimise operational expenses, and align with Environmental, Social, and Governance (ESG) objectives. It also prepares companies for compliance with international climate regulations, such as the EU Green Deal.
Can carbon reduction be achieved without affecting GMP compliance?
Yes, carbon reduction can be achieved without affecting GMP compliance by integrating sustainable design early in facility planning, validating equipment changes, documenting ESG upgrades, and aligning process changes with DQ, IQ, OQ, and PQ protocols.
What are examples of carbon reduction strategies in pharma?
Examples include using low-emission solvents, installing solar panels, switching to hybrid delivery fleets, upgrading to smart HVAC systems, and collaborating with suppliers on ESG performance. Companies like AstraZeneca and GSK have already implemented such initiatives.