Publisher
India Pharma Outlook
published at
June 18, 2025
Designing Sustainable Pharma Facilities: Meeting Environmental Compliance Standards
How can pharmaceutical companies reduce their environmental footprint without compromising on compliance or operational efficiency?
As regulatory pressures and investor expectations intensify, green pharma facility design is no longer optional—it’s a strategic imperative. From ISO 14001 environmental management to GMP-compliant cleanroom planning, pharma manufacturers must now balance sustainability with strict regulatory standards.
This article examines how sustainable pharma facility design can help reduce emissions, lower energy consumption, and streamline audits, while aligning with ISO 14644, GMP, and LEED frameworks. Whether you're planning a new facility or retrofitting an existing one, you’ll discover proven strategies, real-world case studies, and Inotek’s approach to building audit-ready, future-proof pharma plants.
Key Environmental Compliance Standards in Pharma Facility Design
Environmental sustainability in pharmaceutical manufacturing isn’t just optional—it’s becoming a regulatory and operational necessity. Standards such as ISO 14001, ISO 14644, and GMP cleanroom requirements are influencing how facilities are designed and operated. Aligning with these frameworks ensures both regulatory compliance and measurable environmental benefits.
ISO 14001 for Sustainable Pharma Facilities
ISO 14001 outlines requirements for an Environmental Management System (EMS), helping pharmaceutical companies control their environmental impact. It promotes structured processes for waste reduction, energy efficiency, and resource conservation across operations.
Implementing ISO 14001 supports continuous improvement, reduces the risk of non-compliance, and simplifies reporting. For pharma manufacturers, this leads to optimised water and energy usage, safer disposal of chemicals, and more sustainable use of materials, without disrupting core production workflows.
ISO 14644 Cleanroom Design for Green Pharma Manufacturing
Cleanrooms are vital in the pharmaceutical industry, but consume high energy due to air filtration and HVAC requirements. ISO 14644 defines the classification and monitoring of cleanroom performance based on particle concentration and airflow dynamics.
Using this standard helps teams design cleanrooms with efficient airflow systems, smart zoning, and reduced energy waste. Standards such as ISO 14644-1 and ISO 14644-3 enable cleanroom validation methods that promote operational control while reducing excessive ventilation and cooling needs—two significant energy drains in traditional facilities.
Aligning with GMP Guidelines in Sustainable Pharma Facilities
GMP guidelines ensure product safety and quality, but they also have a direct impact on the sustainability of a facility. Cleanroom requirements under GMP include:
- Controlled air quality, temperature, and pressure
- Smooth, cleanable surface
- Defined personnel/material flow paths
- Accurate documentation and traceability
Designing with GMP in mind means better zoning, fewer contamination risks, and more energy-efficient environmental controls. It also simplifies cleaning and maintenance, reducing the use of water, chemicals, and energy over time. When documentation and validation are built into the facility structure, the result is a compliant, efficient, and environmentally friendly pharma operation.
Roadmap to Designing Green, GMP-Compliant Pharma Facilities
Designing a sustainable pharmaceutical facility requires more than just installing green equipment—it demands a clear strategy from start to finish. A structured roadmap ensures that sustainability is embedded at every stage: from site selection and design planning to execution and operational handover. This approach not only improves compliance and cost-efficiency but also helps future-proof facilities against regulatory shifts and environmental risks.
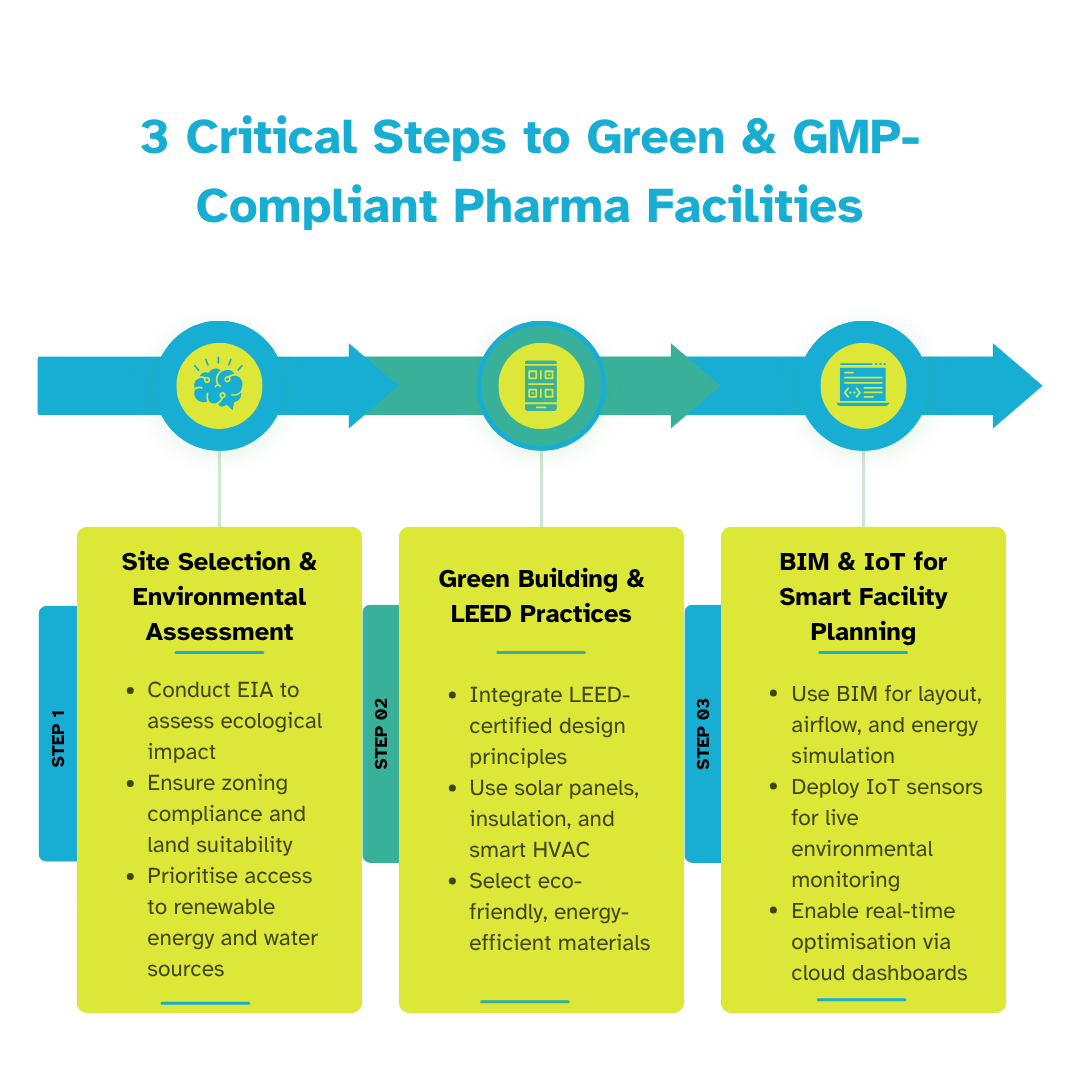
Site Selection & Environmental Impact Assessment
Sustainability starts with location. Selecting the ideal site requires a thorough examination of geography, climate, zoning regulations, and proximity to natural resources. An Environmental Impact Assessment (EIA) is critical in this early stage to evaluate potential harm to ecosystems, water bodies, and surrounding communities.
Proper EIA planning helps avoid legal and environmental setbacks during later stages of the project. It also informs decisions like:
- Proximity to renewable energy sources
- Ease of waste disposal and water recycling
- Land grading and stormwater management
Zoning laws must also be reviewed to ensure the facility can operate within approved environmental thresholds. This early diligence supports smoother construction timelines and long-term operational sustainability.
Green Building & LEED Practices for Pharma Construction
Once the site is secured, the design phase should focus on reducing the building’s environmental load. Applying green building strategies from the outset makes it easier to hit sustainability targets without compromising production capacity.
Key practices include:
- LEED Certification: Ensures the building meets globally recognised green construction standards.
- High-efficiency insulation reduces heating and cooling needs while maintaining temperature stability.
- Solar energy systems: Offset electricity usage and improve ROI.
- Smart HVAC systems: Cut energy waste by optimising airflow and climate control.
Design decisions, such as daylighting, heat recovery units, and the use of eco-friendly materials, further reduce the building’s carbon footprint. According to Scilife, these methods lead to both environmental and financial benefits, making sustainability a cost-effective investment rather than a cost burden.
BIM and IoT in Sustainable Pharma Facility Design
Technology is the backbone of sustainable pharma infrastructure. Building Information Modelling (BIM) enables planners and engineers to simulate the environmental performance of a facility before construction commences. It helps identify design inefficiencies, calculate energy demands, and model waste management systems.
Some key tech enablers include:
- BIM: For 3D/4D simulation of utilities, material flow, and energy performance.
- IoT sensors: For real-time tracking of environmental conditions, power use, and equipment performance.
- Cloud-based dashboards: Help facility managers monitor and optimise resource usage continuously.
United-BIM’s projects show how BIM integration can reduce rework, prevent design clashes, and streamline validation processes—all while keeping sustainability at the centre of facility planning. These tools facilitate easier alignment between pharma teams and construction partners on environmental goals.
Case Studies: GMP-Ready, ISO-Certified Sustainable Pharma Facilities
Designing sustainable pharmaceutical facilities is no longer theoretical. Across the globe, facilities are proving that it's possible to meet strict environmental compliance standards while improving efficiency, reducing costs, and enhancing brand value. The following case studies highlight successful examples where sustainable design aligned with the core principles of ISO 14001, ISO 14644, GMP, and LEED-certified construction, providing practical models for both internal pharma stakeholders and engineering partners involved in facility design and execution.
The International Institute for Sustainable Laboratories (I2SL) features over 80 detailed case studies showcasing high-performance laboratory and pharmaceutical facilities. These projects span academic, private, and government labs that have incorporated:
- Heat recovery systems to reduce HVAC energy demands in cleanrooms
- High-efficiency air handling units that meet cleanroom ISO classifications with lower operational costs
- Water reuse and rainwater harvesting systems
- Renewable energy sources (solar/PV integration)
- Smart lighting and occupancy sensors
- LEED and Green Globes certifications
What stands out is how many of these facilities implemented sustainability without compromising GMP compliance or laboratory performance. For example, several pharmaceutical R&D buildings in the database utilized variable air volume (VAV) fume hoods, which reduced airflow rates without compromising air purity, thereby supporting ISO 14644 compliance while saving energy.
GSK’s Carbon Neutral Laboratory
Located at the University of Nottingham, the GlaxoSmithKline Carbon Neutral Laboratory for Sustainable Chemistry is a pioneering example of how pharma-related R&D spaces can be designed to meet carbon neutrality from the outset—an embodiment of ISO 14001 principles.
Key environmental and compliance-focused features include
- Constructed primarily with timber, a renewable, low-carbon material, reducing embodied carbon compared to traditional concrete or steel.
- Powered by on-site renewable energy sources: solar panels and a biofuel Combined Heat and Power (CHP) system.
- Built to achieve net-zero carbon emissions over 25 years of operation, accounting for both construction and energy use.
- Rainwater harvesting and passive ventilation systems for reduced utility reliance.
- Integrated carbon monitoring to track lifecycle emissions and ensure ongoing compliance with sustainability benchmarks.
The facility is also designed to export excess renewable energy back to the grid, further supporting national sustainability goals. Its approach reflects how sustainability and pharmaceutical compliance (e.g., through responsible material handling, cleanroom support spaces, and energy documentation) can work in tandem.
Genzyme’s LEED Gold Pharma Facility
The Genzyme Center in Cambridge, Massachusetts, designed by Architectural Resources Cambridge (ARC), is a globally recognised example of sustainability within a corporate pharmaceutical setting. The facility earned LEED Gold certification and was once considered one of the most environmentally advanced commercial buildings in the United States.
Standout sustainability and compliance-oriented features:
- A double-skin façade allows natural ventilation and reduces heating and cooling loads by over 40%.
- Reflective ceilings, light shelves, and skylights enable deep daylight penetration, limiting the need for artificial lighting and reducing energy use.
- Graywater systems for toilet flushing and irrigation, cutting water consumption by up to 40%.
- Use of low-VOC (volatile organic compound) paints, adhesives, and materials to maintain indoor air quality—aligning with both LEED and GMP environmental controls.
- Advanced building envelope insulation reduces the overall energy footprint while supporting cleanroom-adjacent lab spaces.
Operationally, the building features centralized automated lighting and climate control, which supports long-term monitoring and documentation, essential for both ISO 14001 audits and internal GMP tracking systems.
These facilities illustrate that with the right planning and design approach, it’s entirely feasible to meet or exceed global environmental standards without sacrificing productivity or regulatory readiness. They provide real, measurable proof that green pharma facility design delivers both compliance and performance, and can be scaled across both R&D and manufacturing environments.
Why Pharma Sustainability Drives Long-Term Value
Sustainability in the pharmaceutical industry is no longer just a CSR checkbox; it has evolved into a strategic business lever. Companies that prioritise green pharma practices gain tangible benefits across operational cost, regulatory ease, and stakeholder trust. With environmental scrutiny increasing globally, integrating sustainability into pharmaceutical facility design is not only about compliance, but also about staying competitive.
According to Pharma’s Almanac, sustainable manufacturing strengthens supply chain resilience, reduces waste across product lifecycles, and enhances global market access, particularly in regions with strict environmental regulations. Facilities designed with ISO 14001, LEED, or GMP-aligned green standards are also more likely to clear audits, meet investor ESG criteria, and minimise disruption during regulatory updates.
Cost Reduction Through Smart Energy Management
Sustainable pharma facilities deliver long-term cost savings through efficient energy systems, particularly in HVAC-heavy environments like cleanrooms. Pharma Focus Europe highlights how retrofitting with:
- High-efficiency chillers and boilers
- Smart LED lighting systems
- Solar power arrays
- Real-time energy monitoring platforms
can reduce energy consumption by up to 25–40%, especially in large-scale production areas.
The return on investment (ROI) for solar installations is often realised within 3–5 years, while HVAC upgrades and automated lighting systems yield both lower utility bills and improved environmental control—a win-win for sustainability and GMP compliance.
Integrating these upgrades during facility construction or renovation avoids later disruption and ensures compatibility with ISO 14644 and GMP zoning requirements, while significantly reducing the carbon footprint.
ESG and CSR: Strengthening Brand with Sustainability
Environmental, Social, and Governance (ESG) criteria are now a key factor for investors, regulators, and even customers in evaluating pharmaceutical companies. Scilife notes that facilities embracing green pharma initiatives not only reduce emissions and waste but also signal long-term stability, ethical governance, and operational maturity.
Examples include:
- Publishing carbon reduction metrics in annual ESG reports
- Gaining LEED or ISO 14001 certifications for manufacturing sites
- Showcasing transparent energy use and water recycling systems in CSR communications
Compliance-Ready Design for Regulatory Peace of Mind
Facilities designed with environmental compliance built-in experience fewer audit complications, faster validation, and stronger documentation trails. Whether it’s via ISO 14001-aligned waste tracking, cleanroom zoning under ISO 14644, or environmentally conscious GMP layouts, a sustainable design foundation simplifies inspections.
Green facility designs often include:
- Pre-planned documentation stations for traceability
- Segregated material/personnel flows to reduce contamination and energy waste
- IoT-based monitoring for temperature, humidity, and air pressure logs
- Low-maintenance surfaces and finishes aligned with cleaning validation protocols
These features reduce operational downtime during audits and enable a faster response to compliance updates from agencies such as the FDA, EMA, and CDSCO. Sustainability and compliance, when co-engineered from day one, create facilities that are resilient, scalable, and audit-ready.
Final Thought
Sustainability in pharmaceutical facility design is no longer just a regulatory expectation—it’s a business-critical necessity. As global compliance standards, such as ISO 14001, ISO 14644, and GMP, tighten, facilities built with environmental compliance at their core offer long-term advantages. From reducing energy and water use to easing regulatory audits and enhancing investor trust through ESG alignment, green pharmaceutical practices directly impact operational resilience and growth.
For pharma companies, designing sustainable facilities isn’t just about meeting today’s benchmarks—it’s about preparing for tomorrow’s scrutiny. For project partners, it's an opportunity to deliver facilities that are audit-ready, resource-efficient, and future-proof from the outset.
Inotek: Your Strategic Partner in Sustainable, GMP-Compliant Pharma Facility Design
The complexities of designing environmentally compliant pharma facilities demand specialised expertise and compliance-focused execution. This is where Inotek steps in as your strategic partner.
We don’t just retrofit green features—we engineer sustainability and regulatory readiness into every project we deliver, aligning with global standards such as ISO 14001, ISO 14644, GMP, and LEED.
Our comprehensive approach includes:
✅ ISO 14001-Integrated Facility Planning: Designs that incorporate waste, water, and emissions control at every level—minimising environmental risk and enhancing audit success.
✅ Energy-Efficient Cleanroom Engineering: Cleanroom layouts validated to ISO 14644 that reduce HVAC loads and energy usage without compromising GMP compliance.
✅ Smart HVAC and Utility Optimisation: BIM and IoT-backed solutions to model, monitor, and reduce facility-wide energy consumption and carbon output.
✅ GMP-Compliant Sustainability Documentation: Environmental validation protocols and documentation frameworks to support CDSCO, EMA, and FDA inspection requirements.
✅ Support for Green Certifications & EIA Approvals: Guidance on achieving LEED, GRIHA, and local green clearances to strengthen brand ESG credentials and project viability.
By partnering with Inotek, pharma manufacturers have achieved:
- Faster EIA and regulatory approval timelines
- Up to 35% reduction in facility-level energy consumption
- Enhanced ESG performance and investor appeal
- Reduced audit deviations tied to environmental non-compliance
While compliance forms the foundation, successful, sustainable facility design must also address growing industry expectations around:
- Long-term environmental stewardship and brand positioning
- Supply chain resilience through resource-efficient utilities
- Digital compliance for real-time environmental monitoring
At Inotek, we ensure your facility isn’t just audit-ready—it’s built for operational excellence, sustainability, and future market access.
Recognised among the Top 10 Pharma Turnkey Contractors & Project Consultants in 2022 & 2025, Inotek helps pharma leaders design, build, and upgrade facilities that meet the strictest GMP and sustainability standards.
📞 Connect with our experts today or visit www.inotek.co.in to schedule a consultation with Mr. Rohit Ochaney.
Whether you're planning a greenfield facility or optimising an existing setup, Inotek ensures your project is compliant, resilient, and future-proof.
FAQs
What are the key environmental standards for pharma facility design?
Key standards include ISO 14001 for environmental management, ISO 14644 for cleanroom classification, and Good Manufacturing Practice (GMP) guidelines for facility compliance. Aligning with these ensures regulatory approval, operational efficiency, and measurable sustainability across energy, waste, and water use in pharma plants.
How does ISO 14644 help in sustainable cleanroom planning?
ISO 14644 enables efficient cleanroom zoning and airflow design, reducing energy consumption in HVAC-intensive environments. It supports the validation of particle control while minimizing over-engineering—helping pharma companies balance contamination control with a lower environmental impact and long-term operational savings.
What role does GMP zoning play in sustainability?
GMP zoning optimises material and personnel flow, reducing cross-contamination and energy-intensive air handling. Efficient zoning reduces HVAC load, streamlines cleaning protocols, and supports ISO-compliant layouts, resulting in sustainable operations that do not compromise on product safety or regulatory readiness.
How can pharma companies reduce energy use in manufacturing facilities?
Pharma firms can reduce energy use by installing smart HVAC systems, adopting BIM-led design, using solar panels, and integrating IoT-based monitoring. These approaches support ISO/GMP compliance while driving down energy costs and enhancing sustainability in high-performance manufacturing environments.


