Publisher
India Pharma Outlook
published at
July 23, 2025
Pharmaceutical Waste Management & Global Compliance
Is your facility’s waste management system ready for an audit?
With growing pressure from global regulators like the EPA, EU WFD, and CPCB, non-compliance in pharmaceutical waste management can lead to fines, shutdowns, and reputational loss.
In this guide, you’ll learn how to:
- Identify and segregate hazardous and non-hazardous pharmaceutical waste
- Meet international environmental regulations (RCRA, WFD, CPCB)
- Implement ISO 14001-aligned systems with digital tracking and documentation
Whether you're upgrading an existing facility or planning a new build, this article gives you actionable steps to ensure your waste systems are compliant, sustainable, and audit-ready.
Understanding Waste Generation in Pharma Facilities
Pharmaceutical facilities generate complex waste streams throughout manufacturing, packaging, and quality control processes. These can include:
- Hazardous chemical waste (e.g., solvents, APIs)
- Expired or recalled medications
- Cytotoxic and genotoxic waste
- Biohazardous materials from labs or cleanrooms
- Packaging waste contaminated with product residues
According to ScienceDirect, poor segregation or documentation of these streams can lead to non-compliance, environmental risk, and process inefficiencies. Even trace levels of improperly handled pharmaceutical waste can leach into soil or water, leading to regulatory action and public backlash.
The challenge for both facility teams and project consultants is to plan waste pathways that prevent cross-contamination, support regulatory documentation, and streamline disposal logistics, right from design.
Key Global Regulations for Pharma Waste Management
Pharmaceutical waste regulations differ by region, but they all aim to ensure:
- Safe handling and tracking of hazardous substances
- Proper documentation from waste generation to disposal
- Environmental protection and public health assurance
As outlined by Adragos Pharma, global pharma firms and their project partners must embed waste compliance into both operational SOPs and facility design strategies.
US: Resource Conservation and Recovery Act (RCRA)
In the United States, pharmaceutical waste is governed under the RCRA Subpart P for hazardous waste pharmaceuticals. Key compliance requirements include:
- Classification of hazardous vs. non-hazardous pharmaceutical waste
- Manifesting: All waste must be tracked via a manifest system from generator to TSDF (Treatment, Storage, and Disposal Facility)
- Training mandates: All personnel involved must be trained on RCRA-specific handling procedures
According to the EPA, these rules reduce mismanagement risks and ensure environmental safety. Design teams and compliance leads must ensure that pharma facilities provide secure storage areas, tamper-proof containers, and access-controlled segregation zones.
EU: Waste Framework Directive (WFD)
The Waste Framework Directive (Directive 2008/98/EC) is the backbone of pharmaceutical waste regulation in Europe. Its key principles include:
- Cradle-to-grave responsibility: Waste producers are accountable until final disposal
- Waste hierarchy: Emphasis on prevention, reuse, recycling, recovery, and then disposal
- Extended Producer Responsibility (EPR): Companies may be required to take back or finance the treatment of their products at end-of-life
Per the European Commission, construction teams working on pharma plants must design closed-loop waste pathways, allow onsite segregation, and enable real-time waste data tracking to support reporting and audit requirements.
India: Biomedical Waste & CPCB Norms
In India, pharmaceutical waste—particularly from formulation plants and healthcare facilities—is regulated by the Biomedical Waste Management Rules, 2016, and the CPCB 2018 Guidelines. Key mandates include:
- Segregation at source using colour-coded bins (e.g., yellow for anatomical waste, red for contaminated plastics)
- Disinfection protocols for reusable containers
- Daily records and manifesting of biomedical waste quantities
As per CPCB guidelines, it is essential to ensure waste is handed over only to authorised Common Biomedical Waste Treatment Facilities (CBWTFs).
To remain compliant, pharma plants must include designated biomedical waste rooms, controlled bin movement routes, and clear SOPs for packaging, labeling, and weighing waste, with training built into onboarding and annual review cycles.
Step-by-Step Waste Compliance Roadmap for Pharma Manufacturers
Effective pharmaceutical waste compliance isn’t about reacting to violations—it’s about building systems that proactively address regulatory expectations. Below is a three-step roadmap that helps manufacturers and facility designers create audit-ready and environmentally compliant waste management systems.
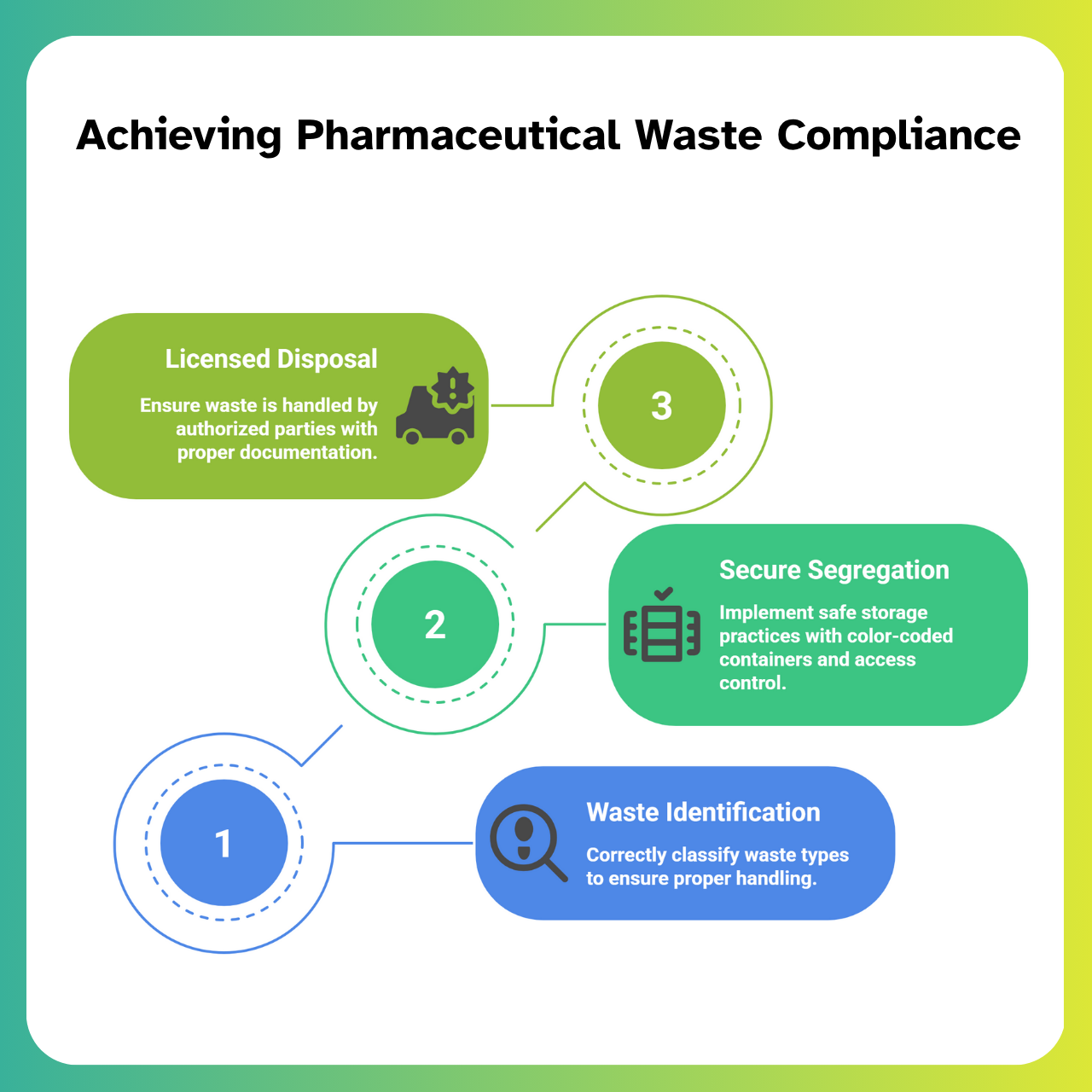
Step 1: Waste Identification & Classification
Before anything can be segregated or disposed of, waste must be correctly identified and categorised.
Per theEPA’s hazardous waste management standards, facilities must classify pharmaceutical waste as:
- Hazardous waste pharmaceuticals (HWPs) – e.g., chemotherapy agents, expired controlled substances
- Non-hazardous pharmaceuticals – e.g., vitamins, saline solutions
- Biohazardous waste – e.g., sharps, contaminated gloves, or PPE
- General/non-pharma waste – e.g., cartons, outer packaging
Accurate classification ensures downstream processes like manifesting, labelling, and treatment follow the correct compliance path. Errors at this stage can trigger audit failures or improper treatment penalties.
Operational Tip: Tag bins at the source with classification codes and integrate these into your digital inventory system.
Step 2: Secure Segregation & Storage
Once waste is identified, it must be immediately routed to the appropriate container under safe storage conditions.
As outlined in the WHO’s Safe Disposal Guidelines, pharma facilities should follow these practices:
- Colour-coded containers: Yellow for cytotoxic, red for biohazards, blue/black for non-hazardous waste
- Clear signage: Use large, multilingual, and pictogram-based labels
- Temperature control: Store heat-sensitive pharmaceuticals in climate-controlled environments until collection
- Access restriction: Storage areas should be secured and accessible only to trained personnel
This step also includes maintaining logs for bin weight, fill date, and handler signatures.
Design Insight: Consider dedicated waste corridors and ventilation-separated storage areas during facility planning to support safe interim holding.
Step 3: Licensed Collection & Disposal
The final step is ensuring that the waste leaves your facility in compliance with both national laws and international best practices.
As per EPA and WHO recommendations, pharmaceutical companies must:
- Use authorised third-party waste handlers: Verify licenses and certifications before contracting
- Maintain manifests: Track waste from site exit to final treatment
- Ensure proper treatment: Confirm that the disposal method—incineration, encapsulation, chemical neutralisation—matches the waste classification.
Documentation must include treatment certificates, pick-up logs, and proof of destruction.
Compliance Insight: Regulators frequently audit the manifest chain. Any break in the chain or missing disposal proof can lead to fines or repeat inspections.
✅ This 3-step roadmap, when integrated into facility design, staff training, and digital documentation systems, ensures a smooth path to compliance and prevents costly operational oversights.
The Real Cost of Non-Compliance in Waste Handling
For pharmaceutical manufacturers, non-compliance with waste disposal regulations isn’t just a paperwork issue—it directly impacts operational continuity, audit performance, and brand credibility.
While regulations vary by region, the consequences of improper classification, storage, or disposal of pharmaceutical waste are consistently severe. From fines and facility shutdowns to reputational damage, the cost of non-compliance can far outweigh the investment in compliant infrastructure and processes.
Legal and Financial Penalties
Regulatory bodies such as the EPA, FDA, and CPCB have the authority to impose significant fines for non-compliant waste practices.
According to MedSharps, common violations include:
- Improper labelling or lack of hazard warnings
- Failure to segregate hazardous and non-hazardous waste
- Absence of manifest documentation or treatment certificates
- Storage beyond permitted time limits
Example: In one case, a US-based pharmaceutical facility was fined over $150,000 for improper disposal of hazardous compounds, with additional penalties linked to incomplete documentation and employee mismanagement.
These costs are often compounded by remediation expenses, legal consultations, and repeated inspections.
Takeaway: Integrating compliant disposal systems into facility design—from colour-coded waste zones to digital manifest logs—reduces legal exposure and audit backlogs
Reputation and Audit Risk
Beyond financial loss, non-compliance damages long-term trust.
Daniels Health reports that improper waste handling can jeopardise:
- GMP certification status
- Regulatory audit outcomes (FDA, EMA, WHO)
- Partnership opportunities with global buyers or investors
Repeated violations often trigger risk-based inspections, increased frequency of regulatory oversight, and internal resource diversion to crisis management.
In addition, violations involving environmental harm, such as improper incineration or waste leaching into the public water source, can trigger public backlash, media scrutiny, and social licence loss.
For project teams, this reinforces the need to build waste compliance into every phase—from design planning and HVAC zoning to SOP development and staff induction.
When waste compliance is treated as a one-time checklist item, it creates systemic vulnerability. The real cost of non-compliance isn’t just financial—it’s operational disruption, legal complexity, and reputational erosion.
Pharma Waste Management Best Practices
Staying compliant is essential, but leading pharmaceutical companies are now focusing on sustainability-driven waste protocols that go beyond regulatory checklists. These best practices not only minimise environmental harm but also help streamline operations, reduce risk exposure, and improve readiness for audits.
By applying tech-enabled tracking, clear labelling protocols, and continuous staff training, pharma manufacturers can build robust waste management systems that scale with their operations and meet both internal and external expectations.
Hazardous vs Non-Hazardous Streams
Understanding the distinction between hazardous and non-hazardous waste is foundational to any pharmaceutical waste strategy.
According to VLS Environmental Services:
- Hazardous waste requires stricter handling, labelled containers, secure storage, and specialised disposal (e.g., incineration for cytotoxic materials).
- Non-hazardous waste, such as packaging or certain OTC drugs, may go through general or recycling streams, but still needs segregation to avoid cross-contamination.
Improper mixing of these streams can contaminate entire disposal batches, triggering regulatory non-compliance and unnecessary cost escalations.
Best practice: Design physical waste flow pathways in the facility with colour-coded signage, floor demarcations, and separate drop-off points for hazardous vs. non-hazardous bins.
Smart Waste Tracking Systems
Waste compliance improves significantly when manual tracking is replaced with smart digital systems.
As highlighted by Indaver:
- IoT-enabled barcoding allows real-time bin-level traceability.
- Cloud-based logs create tamper-proof documentation for regulatory inspection.
- Automated alerts can flag delays, misclassification, or missed pickups.
These digital tools reduce manual errors, simplify audits, and ensure that all disposal events are logged with time stamps, handler data, and waste category identifiers.
Design insight: Smart bins with QR codes and RFID tagging should be included in layout planning, especially for large, multi-zone pharma manufacturing units.
Training & SOP Adherence
Even with advanced systems in place, staff behaviour is the key to ensuring compliance.
As per Sharps Medical Waste Services, ongoing training and well-documented SOPs help:
- Reduce accidental mix-ups and disposal errors
- Improve understanding of compliance deadlines and protocols
- Keep all staff aligned with the latest regulatory changes
Best-in-class pharma plants conduct annual refreshers, maintain digital SOP repositories, and test compliance knowledge through internal audits.
Implementation tip: Integrate compliance training into onboarding, annual review cycles, and validation SOPs.
By combining clear classification systems, real-time tracking tools, and continuous training, pharmaceutical companies can build resilient waste compliance frameworks that are not only audit-ready but also environmentally responsible.
Documentation & Audit-Ready Recordkeeping in Pharma Waste Management
For pharmaceutical companies, proper waste disposal alone isn’t enough—documenting the process in a traceable, verifiable, and audit-ready manner is critical for GMP compliance and regulatory approval. Digital logs and structured systems ensure that your waste management program aligns with environmental regulations and industry best practices.
As noted by JAF Consulting, documentation lapses are among the most common causes of compliance failures during inspections. Missing, inconsistent, or inaccessible records can lead to regulatory observations, repeat audits, or even facility shutdowns.
Well-structured waste documentation systems ensure that:
- All disposal events are traceable
- Regulatory checks are passed without delays
- Internal compliance teams are equipped to conduct preventive reviews
Mandatory Record Types
A strong documentation framework begins with capturing the right records consistently and without gaps.
Essential documentation includes:
- Bin usage logs – Tracking waste volume, classification, and disposal timelines
- Shipping manifests – Confirming transporter details, pick-up times, and disposal route
- Treatment certificates – Issued by authorised waste handlers after destruction
- Incident reports – Logged for any deviations, spillage, or protocol violations
- Staff training records – Documenting completion of SOP modules and refresher sessions
Best practice: Store these records digitally in secure, time-stamped formats with version control, and ensure they’re easily retrievable during audits.
How to Prepare for Inspections
Audit readiness is not a one-time task—it’s an ongoing operational mindset.
JAF Consulting recommends that pharma manufacturers and their project partners implement the following:
- Internal documentation reviews – Scheduled monthly or quarterly, depending on batch volume
- Mock audits – Run by QA or compliance teams to simulate inspection scenarios
- Real-time logging – Ensure bin usage, pick-ups, and destruction events are updated without delay
- Pre-audit readiness kits – including disposal certificates, SOP versions, and training logs in one access-controlled location
Facilities that maintain structured, searchable, and up-to-date documentation are better positioned to satisfy regulators like the FDA, EMA, or CPCB during spot checks.
Design insight: Include audit-ready document storage zones (physical and digital) in facility planning. These should be accessible to QA leads but protected from general plant access.
Environmental Certifications and Continuous Compliance
In today’s regulatory environment, achieving compliance isn’t enough—sustaining it over time through certified systems is what separates operationally strong pharma companies from the rest. Environmental certifications like ISO 14001 are globally recognised tools that help manufacturers meet both legal obligations and GMP-aligned sustainability goals.
An Environmental Management System (EMS) structured under ISO 14001 helps pharma companies reduce environmental impact, improve internal controls, and demonstrate ongoing commitment to safe waste handling and resource efficiency.
For pharma-construction teams, integrating EMS-compatible features into plant design from the start, such as energy-efficient layouts, closed waste loops, and a traceability system, makes long-term certification far more achievable.
ISO 14001 & EMS for Pharma Ops
ISO 14001 provides a structured framework that allows pharmaceutical manufacturers to:
- Identify environmental risks associated with operations
- Implement controls that reduce waste and emissions
- Ensure legal compliance with environmental regulations across jurisdictions
- Win stakeholder trust by demonstrating transparency and accountability
The EMS follows a Plan-Do-Check-Act (PDCA) cycle, making it dynamic and capable of evolving with changes in regulation, operations, or technology.
For pharma, this means a direct contribution to:
- GMP-linked hygiene and waste protocols
- Preparedness for environmental audits (FDA, EMA, CPCB)
- Cost savings via efficient energy and waste resource use
Strategic tip: Align your EMS with both ISO 14001 and local waste mandates (e.g., RCRA, CPCB) to create a unified compliance framework that reduces duplication and audit friction.
Ongoing Monitoring & Continuous Improvement
Certification is not the end goal—maintaining and improving your EMS is where lasting value lies.
ISO 14001 expects organisations to:
- Define environmental KPIs (e.g., waste reduction targets, energy usage, incident rates)
- Conduct internal audits to check if processes meet EMS goals
- Review lifecycle impacts of facility operations and modify SOPs accordingly
- Document and act on findings through continual improvement logs
Implementation tip: Use real-time dashboards to track waste volumes, bin status, and emissions. Regularly update SOPs as regulations or facility layouts change.
For pharma teams and construction partners, embedding these practices into daily operations helps build a culture of accountability and sets the foundation for long-term regulatory and sustainability success.
Why Waste Compliance is a Strategic Advantage in Pharma
When built into design and operations, pharmaceutical waste management becomes a strategic asset, not a compliance burden. Waste compliance in pharmaceutical manufacturing is no longer an isolated task managed by the EHS team—it’s a cross-functional responsibility that spans operations, quality, engineering, and leadership. Regulatory scrutiny is increasing, and facilities are expected to maintain not just clean records but clean systems.
Organisations that invest in robust documentation, digital tracking, smart waste classification, and continuous training are not only reducing their risk exposure—they're building plants that are sustainable, audit-ready, and globally respected.
The next step is clear: move from reactive compliance to proactive integration.
Inotek: Your Strategic Partner in Pharmaceutical Waste Management Compliance
The complexities of pharmaceutical waste management demand specialised expertise and compliance-focused execution. This is where Inotek steps in as your strategic partner.
We don’t just build waste rooms; we engineer integrated, regulation-ready systems into every facility we deliver—aligned with global standards like EPA (RCRA), EU WFD, CPCB, and ISO 14001.
Our comprehensive approach includes:
Design-to-Disposal Waste Flow Engineering:
Purpose-built segregation zones, HVAC zoning, and contamination-free waste corridors for secure, compliant handling from source to storage.
Digitised Tracking & Documentation Systems:
Real-time QR-based monitoring, manifest logging, and cloud-based SOP repositories to streamline inspections and meet audit expectations.
ISO 14001-Aligned Environmental Management Systems (EMS):
Lifecycle-based design principles that reduce environmental footprint while meeting sustainability and GMP-linked regulatory goals.
Smart IoT Bins & Alert Mechanisms:
Sensor-driven waste bins that enable real-time alerts for overflow, delayed pickups, and misclassification, reducing compliance risks.
Training & SOP Integration:
Built-in protocols for onboarding, role-based training, and digital SOP access to ensure team-wide waste compliance readiness.
By partnering with Inotek, pharma manufacturers have achieved:
- Faster facility commissioning with built-in waste compliance features
- 100% documentation pass rates in GMP and environmental audits
- Up to 25% reduction in operational waste handling costs
- Stronger alignment with ISO 14001 and CPCB sustainability targets
While compliance forms the foundation, successful pharmaceutical waste management must also address growing industry expectations around:
- Environmental sustainability and ESG reporting
- Digital traceability and real-time monitoring
- Multi-jurisdictional regulation alignment and audit-readiness
At Inotek, we ensure your waste management systems aren’t just compliant—they’re designed for long-term environmental and operational excellence.
Recognised among the Top 10 Pharma Turnkey Contractors & Project Consultants in 2022 & 2025, Inotek helps pharma leaders design, build, and upgrade facilities that meet the strictest GMP and sustainability standards.
📞 Connect with our experts today or visit www.inotek.co.in to schedule a consultation with Mr. Rohit Ochaney.
Whether you're planning a greenfield facility or optimising an existing setup, Inotek ensures your project is compliant, resilient, and future-proof.
FAQs
What is pharmaceutical waste management?
Pharmaceutical waste management refers to the structured process of handling, classifying, storing, and disposing of pharmaceutical waste to meet health, safety, and environmental regulations.
Why is proper pharmaceutical waste management important?
It ensures environmental protection, prevents regulatory fines, and supports audit-readiness under global standards like RCRA, CPCB, and ISO 14001.
What are the key environmental regulations pharma companies must follow?
These include the Resource Conservation and Recovery Act (RCRA) in the US, the Waste Framework Directive (WFD) in the EU, and the Biomedical Waste Rules (2016) in India.
How can pharma companies improve their waste management systems?
By using IoT-enabled tracking, training staff regularly, implementing ISO 14001 Environmental Management Systems, and ensuring proper classification and storage infrastructure.