Publisher
Inotek
published at
November 12, 2025
How Digital Twins Cut Engineering & Validation Time by 40–70% in Pharma Manufacturing
Why do so many pharma manufacturing projects struggle to meet their launch timelines? The answer lies in the layers of complexity across engineering, commissioning, and validation. Lengthy design iterations, late discovery of automation errors, and repetitive documentation cycles often push projects beyond schedule and budget. Traditional Computer System Validation (CSV) methods, built on manual and paper-heavy testing, create compliance bottlenecks that drain both time and resources.
Today, forward-looking pharma companies are overcoming these challenges through digital twin pharma manufacturing. By simulating systems, processes, and control logic in a virtual environment before execution, teams can detect design flaws early, test automation logic safely, and optimise system performance in advance. This proactive, GMP-compliant approach has been proven to reduce engineering and validation time by 40–70%, accelerating facility readiness without compromising regulatory assurance.
What Is a Digital Twin and How Does It Work?
A digital twin is a dynamic virtual replica of a physical asset, process, or manufacturing system. In pharma manufacturing, it can represent anything from individual process skids and HVAC systems to clean utilities or entire production suites.
Unlike static 3D models, a digital twin continuously mirrors the behaviour, control logic, and performance data of its real-world counterpart. This allows engineering and automation teams to simulate performance before installation, test control logic, run alarm checks, and detect deviations early. By identifying issues before hardware deployment, teams can minimise rework, ensure GMP compliance, and move through commissioning and validation with confidence.
Types of Digital Twins in Pharma Manufacturing
Design Twin
A 3D engineering model that visualises facility layout, equipment placement, and piping geometry. It supports clash detection, cross-discipline coordination, and design reviews among mechanical, electrical, and automation teams, reducing costly design errors before build.
Process Twin
A virtual simulation of key manufacturing processes such as mixing, filtration, or filling. Process twins help engineers optimise cycle times, material usage, and energy efficiency. They enable process adjustments and scenario testing long before validation begins.
Control Twin
A digital replica of PLC and SCADA systems that emulates automation logic. It enables virtual Factory Acceptance Tests (vFATs), verifying interlocks, alarms, and fail-safes in a risk-free simulated environment. This step eliminates on-site debugging and accelerates qualification.
Integration with Automation and PLC Systems
Digital twins in pharma are not isolated software tools; they integrate seamlessly with automation and control environments. Platforms such as Siemens TIA Portal, PLCSIM Advanced, and NX-MCD allow engineers to connect virtual equipment to real control logic.
Through this virtual commissioning process, automation teams can simulate full batch sequences, interlocks, and control loops under realistic conditions. The result is faster FAT completion, shorter CQV timelines, and improved collaboration between design, automation, and validation teams.
Why Digital Twins Matter in a GMP Environment?
Good Manufacturing Practice (GMP) demands full traceability and documented assurance of every system modification. Digital twins support this by creating a continuous digital record of changes, simulations, and test results. Version-controlled models, combined with automated audit trails, simplify documentation during validation and audits. They also enhance collaboration among design, automation, and QA teams, reducing miscommunication between engineering and compliance functions. Under the ISPE Pharma 4.0 framework, digital twins serve as a bridge between engineering data and GMP quality systems, ensuring that each decision is backed by traceable evidence and compliant simulation outputs. For plant heads and project managers, this translates into faster approvals, predictable schedules, and fewer surprises during regulatory inspections.
The Time Challenge: Traditional Engineering & Validation Bottlenecks
Delays in engineering and validation remain one of the biggest roadblocks to timely facility readiness. For pharma manufacturers, late commissioning or extended validation not only stalls production but also increases holding costs and risks of compliance gaps. For project management partners, these delays create cascading schedule conflicts, resource overruns, and missed qualification milestones, directly affecting project ROI.
Engineering & Commissioning Delays
Typical capital projects in pharma lose weeks during commissioning when design or control issues surface late, forcing rework and on‑site debugging. Late‑stage changes to facility layout, equipment configuration, or PLC logic drive additional field hours and costs, which could have been avoided through earlier virtual testing. Commissioning itself can bereduced by up to 40% when development and testing are moved into a virtual environment before hardware arrives, highlighting the time currently lost to on‑site issue discovery. Manual validation often consumes 6–12 months, adding further schedule pressure on CQV milestones.
Validation Inefficiencies under CSV
Traditional CSV emphasizes exhaustive documentation and repeated scripted tests, even for low‑risk changes, which inflates timelines and audit backlogs. Industry assessments note that manual CSV of large systems frequently overruns budgets and schedules due to scope creep and repetitive testing, signalling poor effort‑to‑risk alignment. The FDA’s Computer Software Assurance (CSA) guidance promotes a risk‑based approach that "right‑sizes" evidence, allowing exploratory/unscripted testing where appropriate and reserving heavy scripted testing for high‑risk functions. FDA’s public guidance page confirms CSA’s focus on critical thinking, appropriate assurance activities, and establishing just‑enough records to demonstrate fitness for intended use.
The Resulting Impact on Time‑to‑Market
Every week lost in commissioning or CSV pushes back product release, delaying revenue recognition and extending carrying costs for people and capital equipment. Consultancy analyses of pharma operating models show that process and regulatory bottlenecks materially slow launches and raise costs, underscoring the need to digitize assurance and shift testing earlier in the lifecycle.
Digital Twins Effect (40–70% Time Reduction)
Digital twins are proving that time saved in commissioning and validation isn’t theoretical, it’s measurable. Across pharma and other regulated industries, the integration of virtual commissioning, automation simulation, and digital validation tools has consistently reduced project timelines by 40–70%. These improvements directly benefit engineering teams, quality units, and project partners responsible for delivering compliant facilities on schedule.
Industry‑Wide Findings
Across discrete and process industries, virtual commissioning and digital twin programs consistently report large schedule gains: for example, one Siemens case study documents 70% less on‑site commissioning time using Tecnomatix Process Simulate.
Another Siemens case shows a three‑week launch time reduction by validating control logic ahead of build. Sector articles covering life sciences facilities echo these outcomes, noting decreased physical commissioning times and faster ramp‑up when twins are used from design through startup. Siemens’ pharma narrative further links process twins to shorter development cycles and accelerated vaccine time‑to‑market.
How Virtual Commissioning Achieves These Gains
Virtual FATs connect a control twin (PLC/SCADA logic) to a simulated plant, enabling teams to execute interlocks, alarms, abnormal scenarios, and batch sequences off‑site, eliminating weeks of field debugging.
Case evidence shows that shifting tests upstream reduces rework and delivery time while improving reliability. Academic reviews concur: defects caught during emulation significantly reduce time and effort during real commissioning.
Key Stages Impacted
Design Review: Virtual models enable earlier clash detection and cross‑discipline checks, commonly trimming review/approval cycles by ~15–20% as reported in VC frameworks and case syntheses.
FAT/Commissioning: Reported field time reductions range 30–70%, depending on system complexity, with documented examples at 30% (Estun Automation) and 70% (Wipro PARI).
Validation: By providing executable evidence and defect‑free handover, simulation‑based approaches have been shown to speed qualification by ~25–40% in consulting and vendor studies.
ROI Snapshot
Documented benefits include 20%+ cost reductions tied to less rework and shorter commissioning (Estun reports 20% overall cost savings alongside 30% faster commissioning). Several analyses cite up to ~40% faster time to steady‑state when virtual testing is applied end‑to‑end, freeing significant engineering bandwidth. Broader industry reports link digital twins with measurable OPEX and schedule improvements across manufacturing footprints.
Case Studies: Pharma Leaders Leveraging Digital Twins
Digital twin adoption in leading pharmaceutical facilities has moved beyond pilot projects to full-scale operational use. These case studies illustrate how global pharma companies are using digital replicas to streamline engineering, validation, and production. Each example highlights measurable improvements in efficiency, compliance readiness, and collaboration between process, automation, and quality teams.
Johnson & Johnson Innovative Medicine (Belgium)
Johnson & Johnson’s Innovative Medicine division in Geel, Belgium, introduced a Digital Process Twin to modernize a solvent recovery process, enabling simulation-based control tuning and predictive adjustments.
Using Siemens’ model predictive control (MPC) tools, process engineers created a virtual model that allowed real-time optimisation and parameter testing without interrupting production. The initiative achieved a 35% reduction in solvent use, 30% faster process changeovers, and improved process stability across campaigns.
For validation engineers, the digital twin provided traceable datasets and simulated evidence for performance qualification, aligning directly with GMP and CSA principles. This project demonstrated how digital twins can bridge operations, engineering, and quality functions, driving sustainable, compliant performance improvements.
GSK × Siemens × Atos (Eviden): Vaccine Adjuvant Digital Twin
GlaxoSmithKline collaborated with Siemens and Atos (Eviden) to create a Digital Twin of its vaccine adjuvant production process. The twin used machine learning to link critical process parameters (CPPs) with critical quality attributes (CQAs), helping the team predict outcomes and correct deviations before they impacted yield. By integrating real-time process data with simulation insights, GSK’s R&D and tech transfer teams could scale up adjuvant production faster, achieving shorter time-to-clinical-trial supply and better reproducibility.
Siemens confirmed that this approach reduced physical experimentation needs and supported digital validation under the FDA’s CSA guidance, reducing qualification documentation and enabling faster regulatory readiness.
For pharma construction and engineering partners, this collaboration highlights how early integration of simulation in process development leads to smoother commissioning and validation downstream.
Pfizer: CFD‑Based Bioreactor Digital Twins
Pfizer adopted computational fluid dynamics (CFD)-based digital twins using M‑Star GPU simulation software to optimize bioreactor scale-up for biologics. By creating a virtual replica of the mixing and oxygen-transfer environment, the engineering team could evaluate hydrodynamic performance, nutrient distribution, and shear profiles across reactor scales without extensive lab work. This method reduced physical experimentation by over 40%, improved yield predictability, and accelerated the qualification of new production lines. The bioreactor twin also served as a reusable model for ongoing optimisation and regulatory documentation, supporting faster product transfer to commercial facilities.
For global project stakeholders, this represents a model approach for integrating digital twins into biotech facility design and validation, turning simulation data into validated assurance evidence aligned with GMP and CSA expectations.
Implementation Roadmap: Adopting Digital Twins in Pharma Facilities
Implementing digital twins in a pharma facility requires structured planning that balances technology adoption with compliance needs. A clear roadmap ensures stakeholders, from design engineers to QA teams, align on scope, validation strategy, and measurable business outcomes. The following step-by-step framework outlines how organisations can pilot, validate, and scale digital twins while maintaining GMP standards and project efficiency.
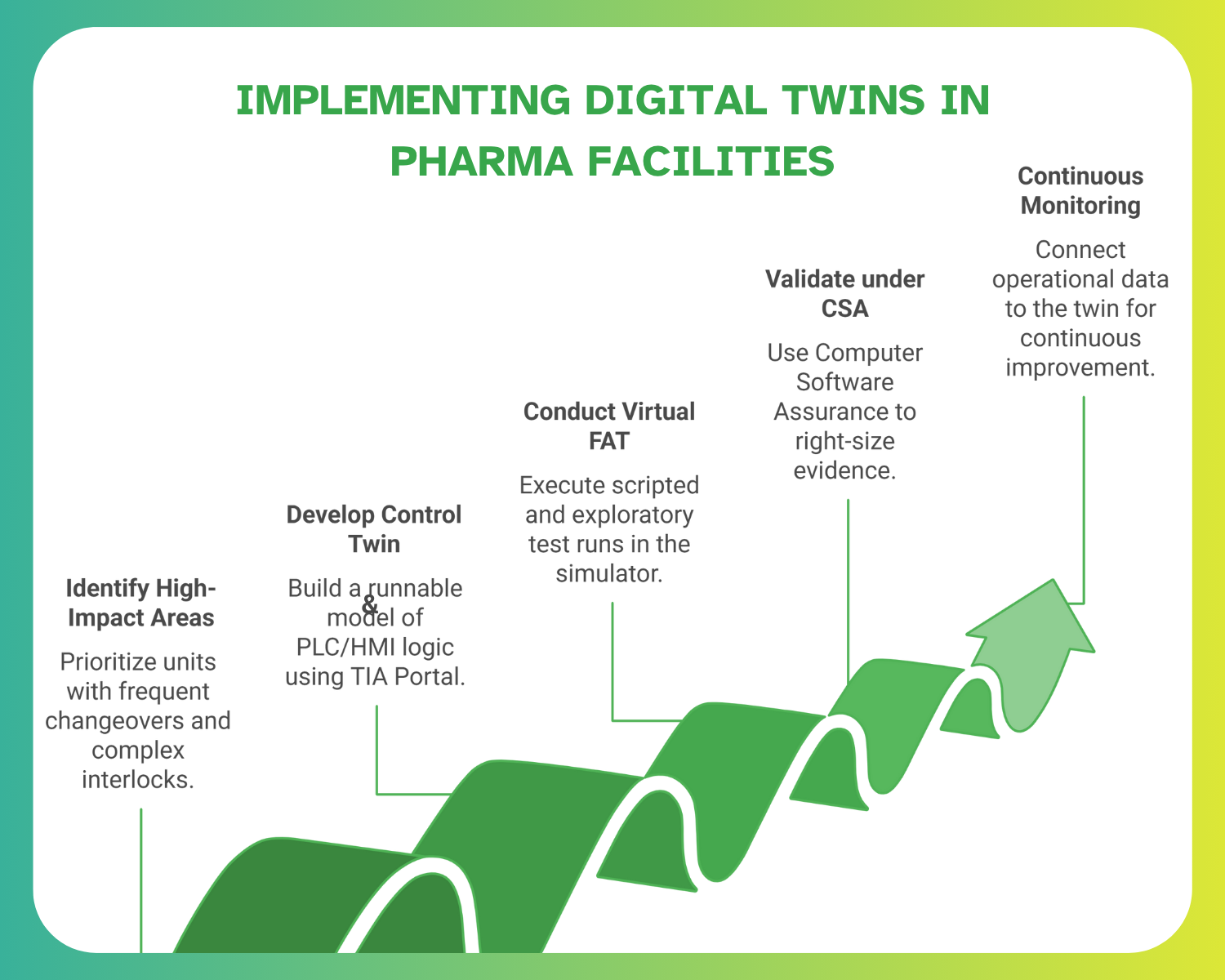
Step 1: Identify High-Impact Areas
Prioritize units with frequent changeovers, tight tolerances, or complex interlocks, e.g., aseptic filling lines, filtration skids, HVAC/clean utility controls, because these areas generate the highest commissioning rework when issues are found late.
Start with a bounded pilot that has clear success metrics (time saved in FAT, number of defects caught pre‑site, documentation hours reduced), reflecting roadmap guidance that recommends focused, low‑risk entries to prove value before scaling. Where remote collaboration is needed, plan for virtual FAT readiness to reduce travel/time costs and minimize onsite FAT scope.
Step 2: Develop the Control Twin
Build a runnable model of PLC/HMI logic using TIA Portal with PLCSIM Advanced so automation code can be executed against a virtual plant before hardware arrives.
Connect the control model to a kinematic/process emulator (e.g., Process Simulate/NX‑MCD or equivalent) to exercise sequences, interlocks, and alarms under realistic timing, which reduces risk and effort at actual commissioning. Research on the synergy between digital twins and virtual commissioning confirms the reusability of models across development, FAT, and operations, improving time‑to‑startup.
Step 3: Conduct Virtual FAT
Execute scripted and exploratory test runs in the simulator to test interlocks, alarms, abnormal scenarios, and batch sequences, eliminating weeks of onsite debugging. For life sciences equipment, virtual FAT improves visibility for cross‑functional teams and reduces the scope of final onsite FAT. Industry guidance shows vFAT programmes can materially cut travel/expense and compress schedules when integrated into commissioning plans.
Step 4: Validate under CSA Framework
Use Computer Software Assurance (CSA) to right‑size evidence: retain targeted scripted tests for high‑risk functions and allow exploratory/unscripted testing for lower‑risk areas, capturing objective evidence of fitness for intended use. Your validation record should link intended use, risk rationale, test activities, results/issues, and conclusions/sign‑offs, enabling reuse of supplier/developer testing where appropriate.
Validation 4.0 resources from ISPE support this model‑based approach by promoting digital tools and earlier testing to keep validation an accelerator, not a bottleneck.
Step 5: Continuous Monitoring & Optimisation
After go‑live, connect operational data to the twin to support deviation investigations and continuous improvement, aligning with Validation 4.0 principles of real‑time verification and data‑driven assurance. Use feedback loops and predictive analytics to refine control strategies and maintenance plans, reducing downtime and improving reliability over time. Periodically review model fidelity and configuration under change control to ensure data integrity and audit readiness as processes evolve.
The Future of Digital Twins in Pharma Manufacturing
The next wave of innovation in pharma manufacturing will be driven by intelligent, data-connected digital twins. As plants evolve toward continuous operations and predictive control, digital twins will serve as the backbone for decision-making, bridging AI, sustainability, and quality initiatives. The following trends illustrate how the technology is shaping long-term competitiveness and compliance readiness.
AI-Enhanced Predictive Twins
AI models embedded in digital twins can flag drift in process parameters and predict deviations before they impact yield, enabling operators to adjust set points or trigger maintenance proactively. Manufacturers report using AI with twins to optimize flexibility, maintenance, and energy consumption in production plants as part of smart manufacturing programmes.
Industry analyses highlight the convergence of AI/ML with digital twins in manufacturing and packaging to reduce downtime and stabilize product quality through predictive control.
Role in Continuous Manufacturing
Digital twins support continuous manufacturing by connecting models to real‑time PAT signals and applying QbD principles to maintain control, a precondition for real‑time release testing (RTRT). FDA’s Q14 guidance further describes multivariate analytical procedures and RTRT concepts that align with model‑based control strategies. ISPE guidance summarizes how PAT in continuous operations is anchored by ICH Q8/Q9/Q10 and can be operationalized via digital tools that sustain process understanding and control.
Sustainability & Energy Efficiency
Facility and utility twins allow teams to simulate HVAC set points, air‑change rates, and equipment scheduling to reduce energy use while meeting cleanroom requirements, cutting both OPEX and carbon impact. Building‑scale studies show that digital twins with BIM/IoT/AI can optimize energy consumption and support predictive maintenance of utility capabilities, directly applicable to GMP facilities. Vendor case narratives also link process twins to shorter development cycles and improved resource efficiency in pharma operations.
Inotek: Your Strategic Partner in Digital Twin Implementation for Pharma Manufacturing
Pharma manufacturing projects are often delayed by long engineering and validation cycles. Traditional commissioning and Computer System Validation (CSV) processes can take months, leading to late product launches and compliance bottlenecks. Digital twin pharma manufacturing solutions now enable project teams to simulate systems, processes, and controls before physical execution. These virtual replicas have been proven to reduce engineering and validation timelines by 40–70%, while maintaining full GMP compliance.
The Complexities of Digital Twin Adoption
The complexities of implementing digital twins in pharma manufacturing demand specialized expertise and compliance-focused execution. This is where Inotek steps in as your strategic partner. We don’t just integrate digital tools; we engineer simulation-driven, validation-ready solutions into every facility we deliver, aligned with global regulatory standards such as those of the FDA, EMA, and CDSCO.
Our Comprehensive Approach Includes:
- Digital Engineering & Virtual Commissioning: Creating model-based engineering workflows and virtual FATs to accelerate commissioning and minimize rework.
- GMP-Compliant Validation under CSA: Implementing Computer Software Assurance (CSA) frameworks that streamline documentation and enhance data integrity.
- HVAC & Utility System Simulation: Designing validated digital twins for cleanroom utilities, ensuring optimal performance, air balance, and energy efficiency.
- Process & Automation Integration: Bridging PLC, SCADA, and MES environments for seamless digital validation and control testing before site execution.
By partnering with Inotek, pharma manufacturers have achieved:
- Up to 70% faster commissioning timelines through virtual validation and automated FATs.
- Reduced compliance risk via validated digital documentation and traceable simulation logs.
- Improved sustainability through energy-optimized design and predictive maintenance modelling.
While compliance forms the foundation, successful digital twin adoption must also address industry expectations around:
- Sustainability and energy efficiency
- Data integrity and digital assurance
- Supply chain resilience and long-term scalability.
At Inotek, every digital initiative is engineered not only to be audit-ready but also to enable operational excellence and lifecycle efficiency across the facility.
Recognized among the Top 10 Pharma Turnkey Contractors & Project Consultants in 2022 & 2025, Inotek helps pharma leaders design, build, and upgrade facilities that meet the strictest GMP and sustainability standards.
📞 Connect with our experts today or visit www.inotek.co.in to schedule a consultation with Mr Rohit Ochaney.
Whether you're planning a greenfield facility or optimising an existing setup, Inotek ensures your project is compliant, resilient, and future-proof.
FAQs
How do digital twins accelerate pharma manufacturing projects?
Digital twins simulate systems, processes, and control logic in a virtual environment before real execution. This early testing reduces design errors, rework, and manual validation cycles, helping pharma teams complete engineering, commissioning, and qualification 40–70% faster while maintaining GMP compliance.
What are the main types of digital twins in pharma manufacturing?
Pharma facilities typically use three types: Design Twins for layout and clash detection, Process Twins for optimising cycle times and resources, and Control Twins for virtual testing of PLC and SCADA logic before commissioning and validation.
How does virtual commissioning support GMP compliance?
Virtual commissioning uses digital twins to test automation sequences and interlocks before site installation. It ensures systems perform as intended, generates audit-ready evidence, and aligns with GMP and CSA guidelines by documenting every simulated test result for traceable assurance.
What is the difference between CSV and CSA in pharma validation?
Traditional Computer System Validation (CSV) relies on extensive manual testing and documentation. Computer Software Assurance (CSA) takes a risk-based approach, using critical thinking, automation, and simulation evidence to prove system fitness, reducing redundant tests and improving validation speed and audit readiness.
How can pharma companies start implementing digital twins?
Begin with high-impact areas such as HVAC, clean utilities, or filling lines. Create control and process twins, conduct virtual FATs, and validate under CSA frameworks. Partnering with experienced turnkey consultants ensures GMP-aligned, risk-based adoption and measurable efficiency improvements.